Percutaneous administration device of bisoprolol
A technology of bisoprolol and drug delivery device, applied in the field of bisoprolol transdermal drug delivery device, can solve problems such as skin irritation, and achieve the effects of reducing skin irritation, suppressing large fluctuations, and improving cohesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
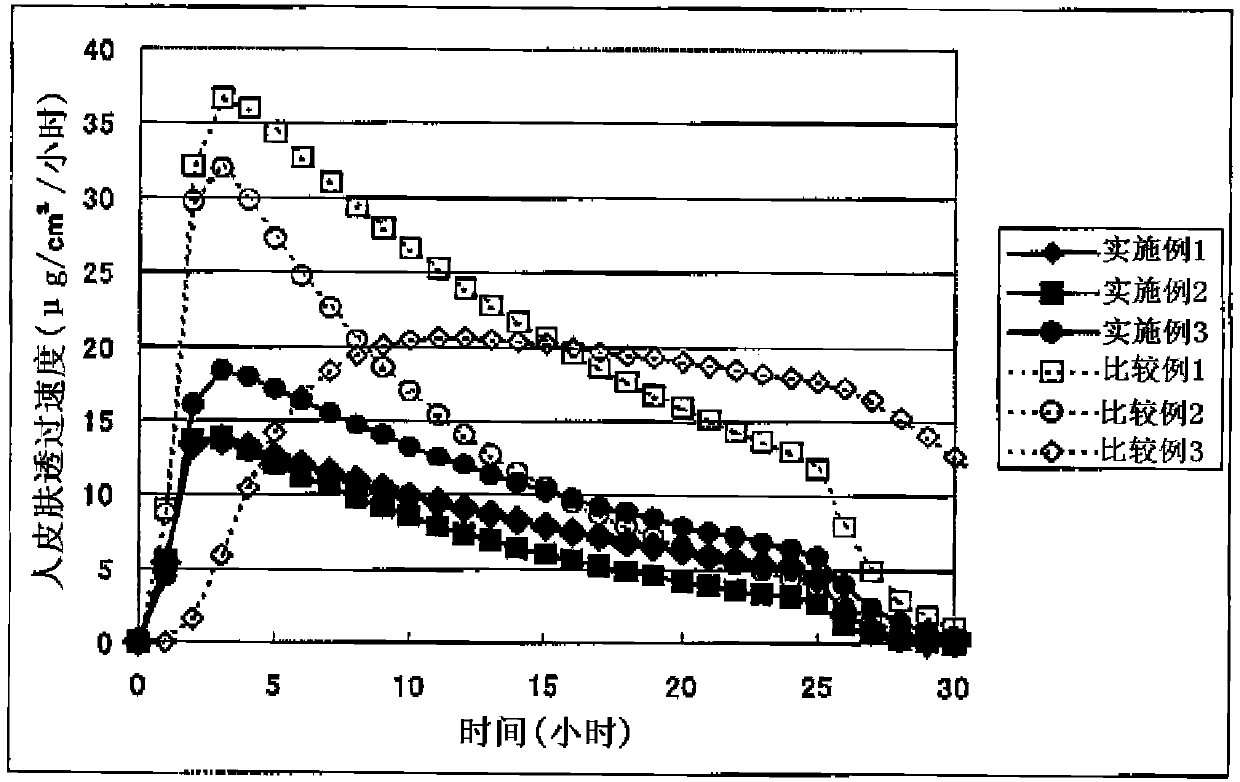
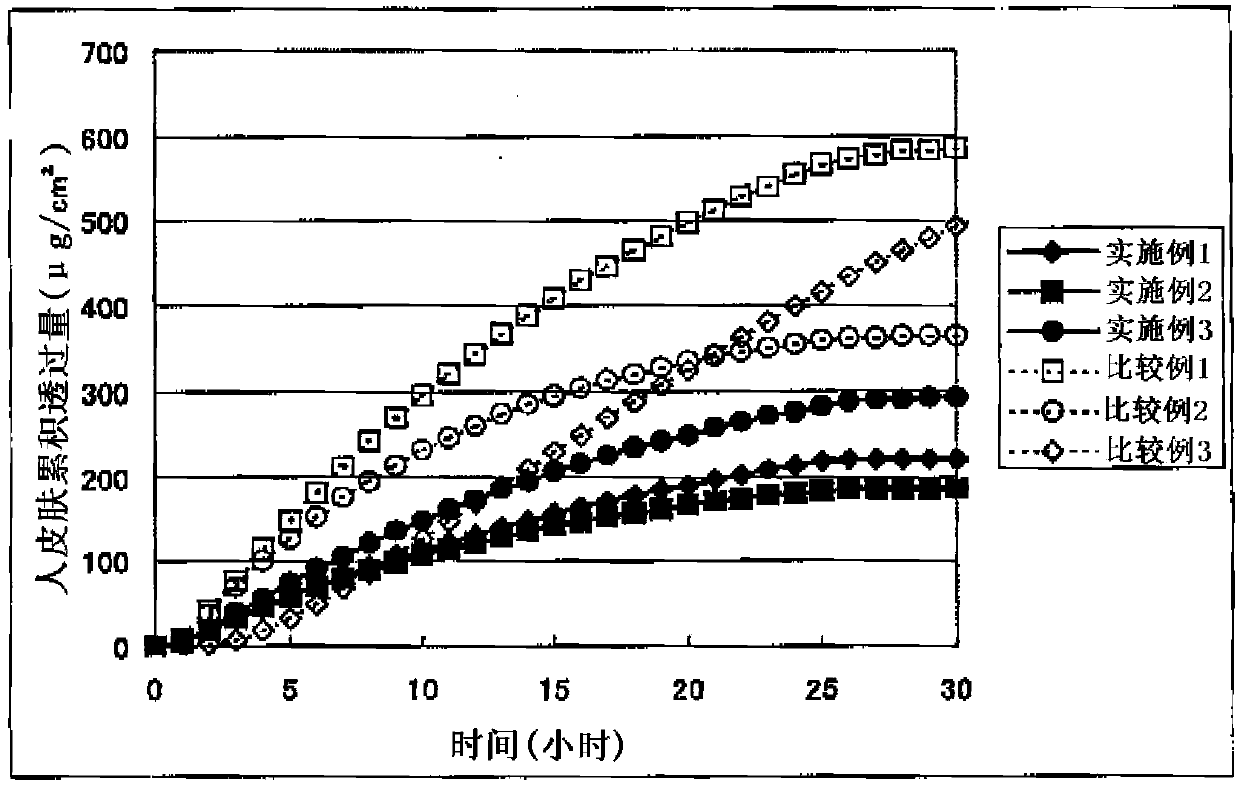
Image
Examples
Embodiment 1 to 2
[0125] A viscous toluene solution of the pressure-sensitive adhesive composition was prepared according to the mixing ratio shown in Table 1; On a pad (thickness 75 μm), which had previously been subjected to a silicone release treatment; it was then dried in a hot air circulation type dryer at 100° C. for 5 minutes, thereby forming a pressure-sensitive adhesive layer. The pressure-sensitive adhesive layer was bonded on a PET film with a thickness of 12 μm, or a PET film with a thickness of 2 μm and 12 g / m 2 A laminated film of PET nonwoven fabric was placed on the nonwoven fabric side, thereby obtaining a sheet-shaped laminate. The liner made of PET of this laminate was peeled off, and several pressure-sensitive adhesive layers having the same composition and thickness as above were laminated on the exposed pressure-sensitive adhesive surface, thereby obtaining Thickness of the pressure-sensitive adhesive layer of the transdermal drug delivery device. The mixing amount of e...
Embodiment 3 and comparative example 1 to 3
[0127] In an inert atmosphere, 70 parts by weight of 2-ethylhexyl acrylate, 20 parts by weight of 2-methoxyethyl acrylate, 10 parts by weight of 2-hydroxyethyl acrylate and 0.2 parts by weight of azobisiso Nitrile was solution-polymerized in ethyl acetate at 60° C., thereby preparing a solution of an acrylic pressure-sensitive adhesive. The acrylic pressure-sensitive adhesive, isopropyl myristate and bisoprolol were uniformly mixed and stirred in a container according to the mixing ratio shown in Table 1; 0.6% by weight (relative to the pressure-sensitive adhesive solid) of ethyl acetoacetate aluminum diisopropionate; and adjust the viscosity with ethyl acetate. The obtained solution was coated on a liner (thickness 75 μm) made of polyethylene terephthalate (PET) which had previously been treated with silicone release in the dried thickness shown in Table 1 ; and then it was dried at 100° C. for 5 minutes in a hot-air circulation type dryer, thereby forming a pressure-sensiti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com