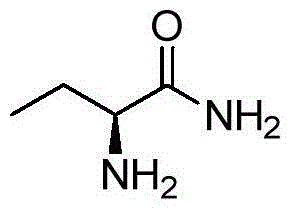
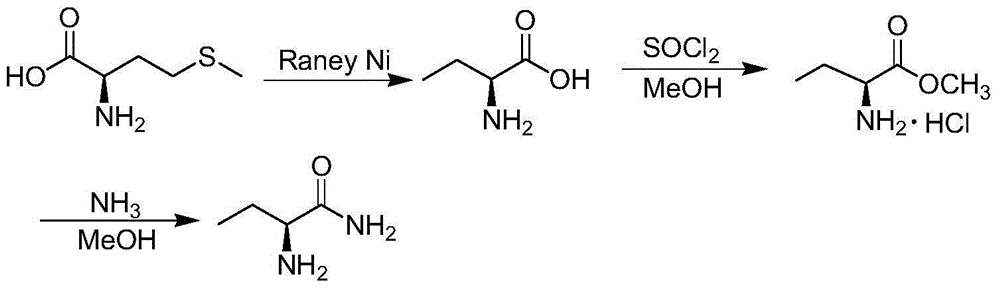
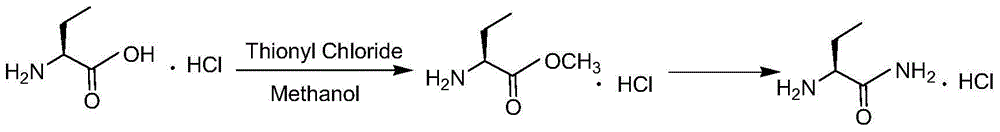
Method for preparing levetiracetam intermediate 2-aminobutyric acid
A technology for aminobutyric acid and intermediates, which is applied in the field of preparation of levetiracetam intermediates, can solve the problem that the S-2-aminobutyramide process route has no literature reports, etc., to prevent polychlorinated products and prevent reactions Faster, less by-product effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]Take butyric acid (120.00g, 1.36mol) and BTC (12.11g, 0.04mol) to form a butyric acid reaction solution with a substance ratio of 1:0.03, and send the butyric acid reaction solution to the first pipeline through a liquid delivery pump chemical reactor (20 meters in length and 8 mm in inner diameter), and at the same time, chlorine gas is introduced into another branch of the first pipeline chemical reactor, and the flow rate in the tube is controlled so that the butyric acid and chlorine gas in the butyric acid reaction solution introduced The ratio of the amount is 1:1.5, the reactor is closed, and the reaction is carried out at a temperature of 110° C. for 10 h. After the reaction, the outflowing reaction liquid enters the rectification reactor, and the fraction at about 90°C (12mmHg) is collected by distillation to obtain 156.67g of 2-chlorobutyric acid, with a yield of 94.0% and a purity of 96.0%. 1 H NMR (DMSO, 500MHz): δ4.43-4.40 (m, 1H), 2.01-1.92 (m, 1H), 1.90-1....
Embodiment 2
[0046] Take butyric acid (120.00g, 1.36mol) and butyric anhydride (6.33g, 0.04mol) to form a butyric acid reaction solution with a substance ratio of 1:0.03, and send the butyric acid reaction solution to the first Pipelined reactor (20 meters in length, 8 millimeters in pipe inner diameter), slowly feed chlorine gas into another branch of the first piped reactor at the same time, control the flow rate in the pipe so that butyric acid and The molar ratio of chlorine gas to substance is 1:1.5, and the reactor is sealed and reacted at a temperature of 110°C for 10 hours. After the reaction, the outflowing reaction liquid enters the rectification reactor, and the fraction at about 90°C (12mmHg) is collected by distillation to obtain 141.67g of 2-chlorobutyric acid, with a yield of 85.0% and a purity of 88.0%.
[0047] Take the above obtained 2-chlorobutyric acid (141.67g, 1.16mol) and ammonium chloride (92.77g, 1.73mol) to form a 2-chlorobutyric acid reaction solution with a mass...
Embodiment 3
[0049] Take butyric acid (120.00g, 1.36mol) and thionyl chloride (4.76g, 0.04mol) to form a butyric acid reaction solution with a substance ratio of 1:0.03, and send the butyric acid reaction solution to In the first pipelined reactor (20 meters in length and 8 mm in inner diameter of the tube), chlorine gas is slowly introduced into another branch of the first pipelined reactor at the same time, and the flow rate is controlled so that the butyric acid in the butyric acid reaction solution introduced The molar ratio of the substance to the chlorine gas is 1:1.5, the reactor is closed, and the reaction is carried out at a temperature of 110°C for 10h. After the reaction, the outflowing reaction liquid enters the rectification reactor, and the fraction at about 90°C (12mmHg) is collected by distillation to obtain 146.67g of 2-chlorobutyric acid, with a yield of 88.0% and a purity of 94.0%.
[0050] Take the above obtained 2-chlorobutyric acid (146.67g, 1.20mol) and ammonium chlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com