Electrolytic solution of rechargeable magnesium cell and application method thereof
An electrolyte and magnesium battery technology, applied in secondary batteries, circuits, electrical components, etc., can solve the problems of complex preparation process, and achieve the effects of simple preparation, high magnesium deposition-dissolution efficiency, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Using N-methylaniline and ethylmagnesium chloride as solutes and tetrahydrofuran as solvent, the configuration is 2mol·L -1 N-methylaniline-ethylmagnesium chloride / tetrahydrofuran electrolyte, wherein the molar ratio of N-methylaniline to ethylmagnesium chloride is 1:1.
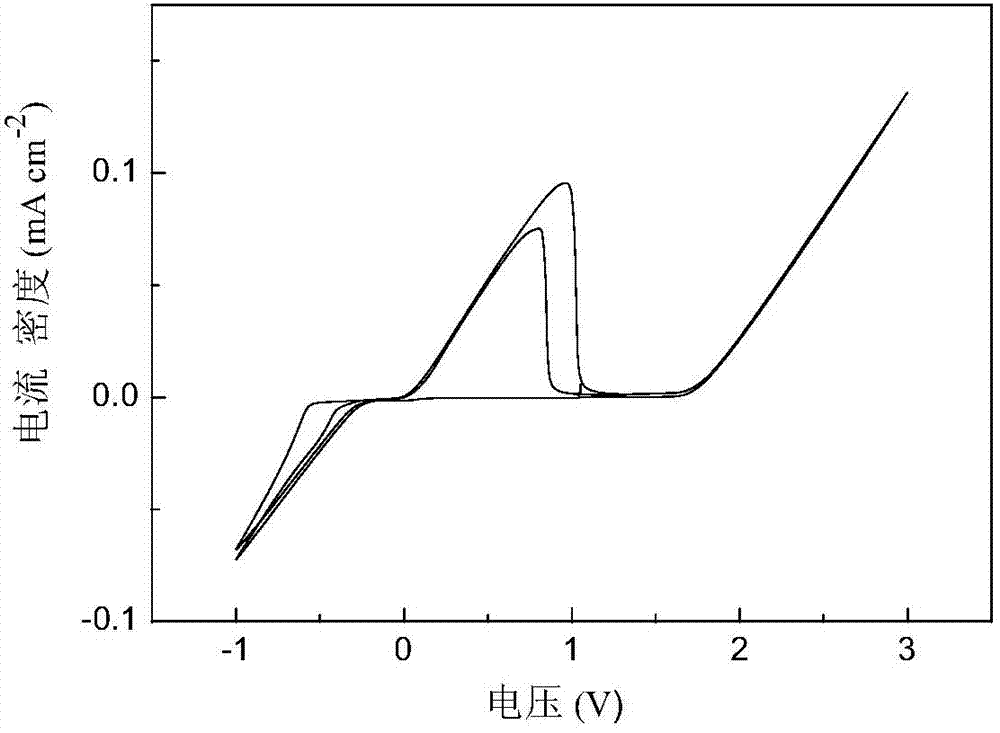
[0034] In the three-electrode tube, platinum is used as the working electrode, and 3mL of 2mol·L is added -1 N-methylaniline-ethylmagnesium chloride / tetrahydrofuran electrolyte, metal magnesium as counter electrode and reference electrode, assembled into a three-electrode system, cyclic voltammetry test in an argon glove box, scanning speed 50mV·s -1 . Cyclic voltammetry results such as figure 1 As shown, the magnesium deposition-dissolution performance is good, and the anodic oxidation decomposition potential of the electrolyte is 1.65V vs. Mg.
Embodiment 2
[0036] Using N-methylaniline and phenyl magnesium chloride as solutes, and tetrahydrofuran as solvent, the configuration is 2mol·L -1 N-methylaniline-phenylmagnesium chloride / tetrahydrofuran electrolyte, wherein the molar ratio of N-methylaniline to phenylmagnesium chloride is 1:1.
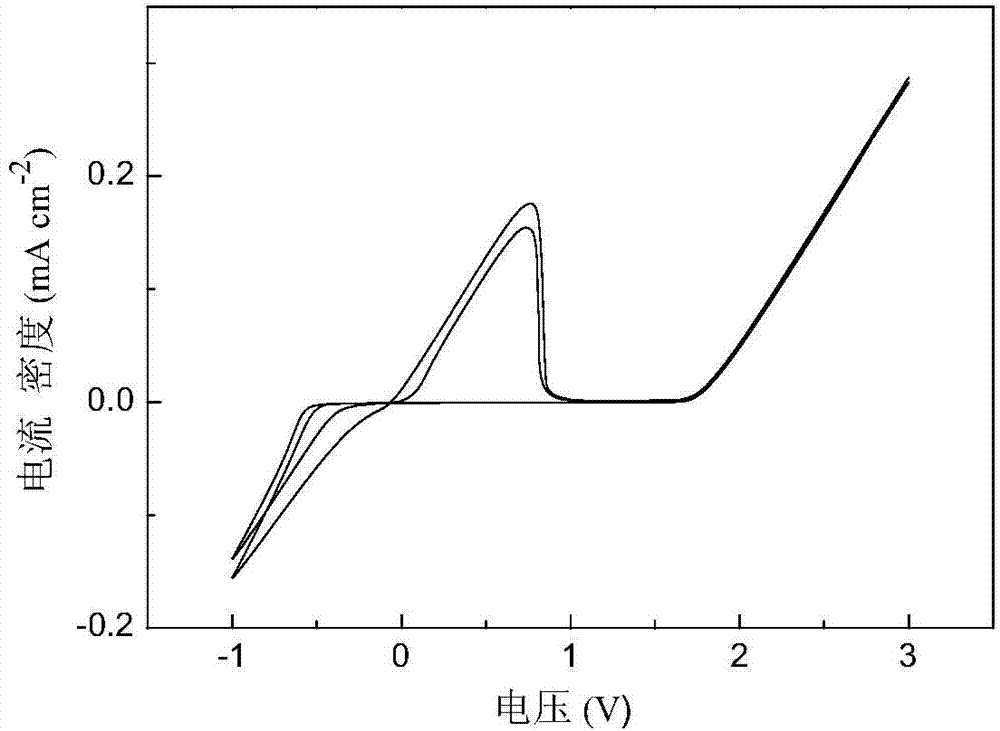
[0037] In the three-electrode tube, platinum is used as the working electrode, and 3mL of 2mol·L is added -1 N-methylaniline-phenylmagnesium chloride / tetrahydrofuran electrolyte, metal magnesium as counter electrode and reference electrode, assembled into a three-electrode system, cyclic voltammetry test in an argon glove box, scanning speed 50mV·s -1 . Cyclic voltammetry results such as figure 2 As shown, the magnesium deposition-dissolution performance is good, and the anodic oxidation decomposition of the electrolyte is 1.65V vs.Mg.
Embodiment 3
[0039] Using N-methylaniline, ethylmagnesium chloride and aluminum trichloride as solutes and tetrahydrofuran as solvent, the configuration is 0.5mol·L -1 N-methylaniline-ethylmagnesium chloride-aluminum trichloride / tetrahydrofuran electrolyte, in which the molar ratio of N-methylaniline to ethylmagnesium chloride is 1:1, and the molar ratio of aluminum trichloride to N-methylaniline It is 1:1.
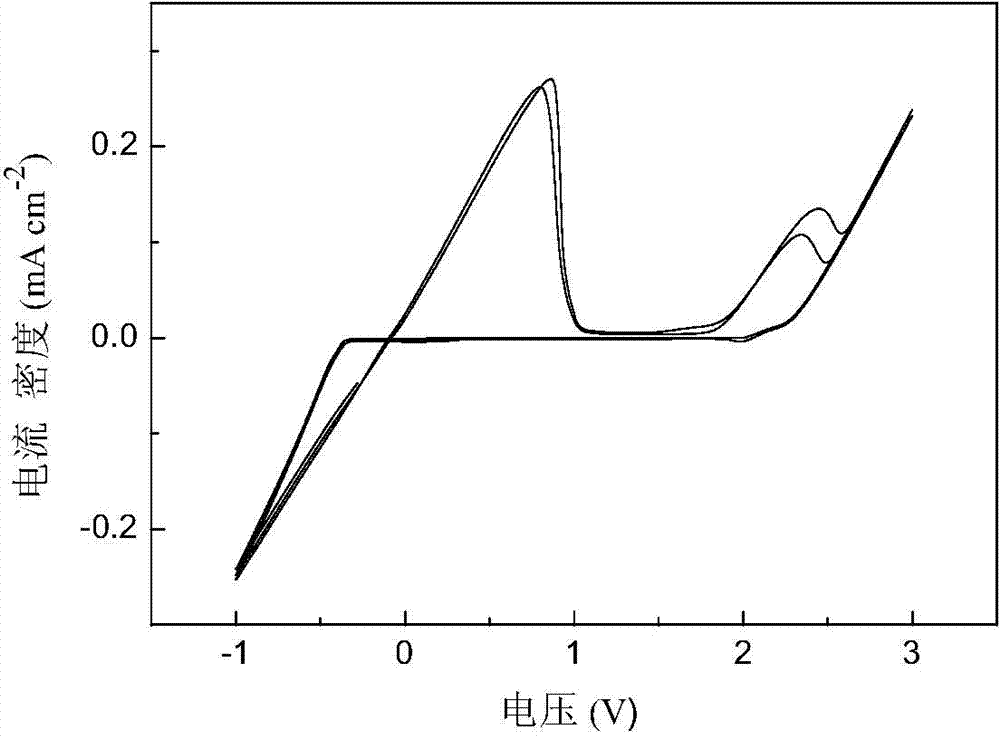
[0040] In the three-electrode tube, platinum is used as the working electrode, and 3mL of 0.5mol·L is added -1 N-methylaniline-ethylmagnesium chloride-aluminum trichloride / tetrahydrofuran electrolyte, metal magnesium as counter electrode and reference electrode, assembled into a three-electrode system, cyclic voltammetry test in an argon glove box, scanning speed of 50mV ·S -1 . Cyclic voltammetry results such as image 3 As shown, the magnesium deposition-dissolution performance is good, and the anodic oxidation decomposition of the electrolyte is 1.8V vs. Mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com