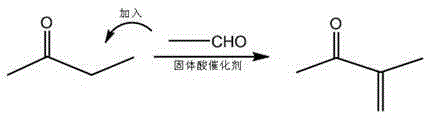
The preparation method of 3-methyl-3-penten-2-one
A technology of pentene and methyl is applied in the field of preparation of intermediate 3-methyl-3-penten-2-one, can solve the problems of high production cost, serious environmental pollution and high equipment requirements, and achieves energy consumption and Low production cost, high yield and purity, and the effect of reducing separation difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Under normal pressure, add 540g methyl ethyl ketone (AR) and 162g American Rohm and Haas dry hydrogen Amberlyst15 catalyst to a 1L glass stirred tank reactor, store 165g acetaldehyde (99%) in the dropping funnel, drop the dropping funnel The lower port is provided with a catheter extending into the bottom of the reactor, and the reaction is heated by a water bath. When the temperature reaches 45°C, acetaldehyde is added dropwise to the reactor, and the dropwise time of acetaldehyde is controlled to be 1.5h, and the reaction is continued after the drop is completed. After 3 hours, the reaction was stopped, and the material obtained after the reaction was analyzed by chromatography. It was determined that the single-pass conversion rate of butanone was 48%, the selectivity of 3-methyl-3-penten-2-one was 92%, and some impurities were generated in the rest.
[0021] The material obtained after the reaction enters the separation unit, distills and recovers butanone and separa...
Embodiment 2
[0023] Under normal pressure, add 540g methyl ethyl ketone (AR) and 162g American Rohm and Haas dry hydrogen Amberlyst35 catalyst to a 1L glass stirred tank reactor, store 275g acetaldehyde (99%) in the dropping funnel, drop the dropping funnel The lower port is provided with a catheter extending into the bottom of the reactor, and the reaction is heated by a water bath. When the temperature reaches 55°C, acetaldehyde is added dropwise to the reactor. The time for adding acetaldehyde is controlled to be 1h, and the reaction is continued for 1h after the drop is completed. Afterwards, the reaction was stopped, and the material obtained after the reaction was analyzed by chromatography to determine that the single-pass conversion rate of butanone was 67%, the selectivity of 3-methyl-3-penten-2-one was 96%, and the rest generated some impurities.
[0024] The material obtained after the reaction enters the separation unit, distills and recovers butanone and separates impurities, a...
Embodiment 3
[0026] In a 5L double-layer glass stirred reactor, add 1800g butanone and 900g LANXESS dry-type LewatitK2620 catalyst, store 1100g of acetaldehyde (99%) in the liquid storage tank, and the double-layer glass stirred reaction passes through the interlayer. Enter hot water for heating, when the temperature reaches 50°C, use a peristaltic pump to input acetaldehyde at a constant speed to the bottom of the reactor, control the input time of acetaldehyde to 2h, then continue to react for 0.5h and then stop the reaction, and analyze the obtained product by chromatography As for the material, it is determined that the single-pass conversion rate of butanone is 82%, the selectivity of 3-methyl-3-penten-2-one is 93%, and the rest generate some impurities.
[0027] The material obtained after the reaction enters the separation unit, distills and recovers butanone and separates impurities, and the purity of 3-methyl-3-penten-2-one obtained after separation is 97.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com