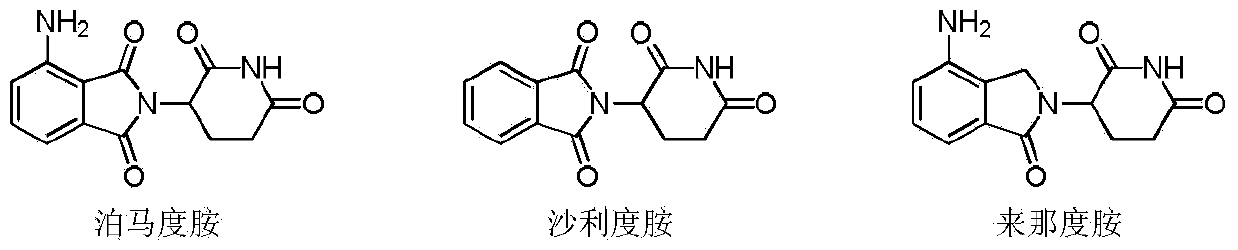
Method for preparing high-purity pomalidomide
A pomalidomide and high-purity technology is applied in the field of preparation of high-purity pomalidomide, which can solve the problems of unmentioned intermediate and product purity, inability to meet the requirements of the pharmaceutical industry, and high requirements for purity and impurity content, Achieve the effect of less waste, low price and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
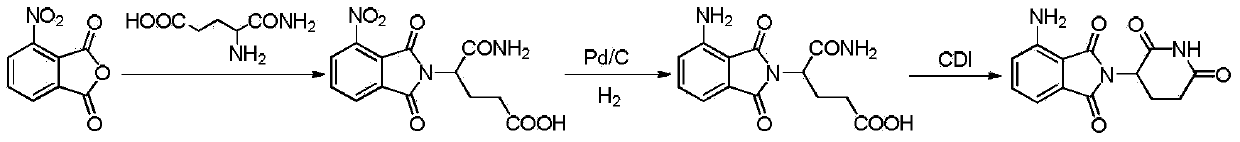
[0030] Add 24.0g (0.124mol, 1.0eq) of 3-nitrophthalic anhydride, 21.5g (0.130mol, 1.05eq) of 3- Aminopiperidine-2,6-dione hydrochloride and 240mL of toluene as a solvent were stirred to form a suspension, then 15.0g (0.148mol, 1.2eq) of triethylamine was added, and the temperature was slowly raised to reflux to separate water, and the reaction solution Turn into a purple suspension, continue to reflux and divide water for 8 hours, HPLC central control: when the content of 3-nitrophthalic anhydride in the raw material is 1 H NMR characterization, showing the following chemical shifts: (DMSO-d6)δ: 11.122(s, 1H), 8.342(d, J=8.1Hz, 1H), 8.229(d, J=7.2Hz, 1H), 8.122( t,J=7.8Hz,1H),5.215(dd,J=12.6,5.4Hz,1H),2.946-2.825(m,1H),2.631-2.571(m,2H),2.093-1.981(m,1H) ;MS(ESI)(m / z):274.4[M+H] + .
[0031] Add 31.7g (wet product 48.2g, 0.105mol, 1.0eq) 3-nitro-N-(2,6-dioxo-3 -piperidinyl)-phthalimide, 1.6g of 10% palladium on carbon and 395mL of 1,4-dioxane as a solvent, stirred to form a...
Embodiment 2
[0033] Add 24.0g (0.124mol, 1.0eq) of 3-nitrophthalic anhydride, 21.5g (0.130mol, 1.05eq) of 3- Aminopiperidine-2,6-dione hydrochloride and 240mL of toluene as a solvent were stirred to form a suspension, then 15.0g (0.148mol, 1.2eq) of triethylamine was added, and the temperature was slowly raised to reflux to separate water, and the reaction solution Turn into a purple suspension, continue to reflux and divide water for 8 hours, and control in the HPLC: when the content of the raw material 3-nitrophthalic anhydride is <0.5%, and the temperature of the reaction solution drops to 15-25°C, add 10.0g N,N'-carbonyldiimidazole (0.062mol, 0.5eq), slowly heat up to reflux and stir for 2 hours, HPLC central control: when the intermediate state content is less than 0.5%, and the temperature of the reaction solution drops to 15-25°C, pump Filter, transfer the filter cake to another 250mL reaction bottle, add a mixture of 60mL methanol and 60mL water to make a slurry for 2 hours, filter...
Embodiment 3
[0036] Add 24.0g (0.124mol, 1.0eq) of 3-nitrophthalic anhydride, 21.5g (0.130mol, 1.05eq) of 3- Aminopiperidine-2,6-dione hydrochloride and 240mL of toluene as a solvent were stirred to form a suspension, then 18.8g (0.186mol, 1.5eq) of triethylamine was added, and the temperature was slowly raised to reflux to separate water, and the reaction solution Turn into a purple suspension, continue to reflux and divide water for 8 hours, and control in the HPLC: when the content of the raw material 3-nitrophthalic anhydride is <0.5%, and the temperature of the reaction solution drops to 15-25°C, add 10.0g N,N'-carbonyldiimidazole (0.062mol, 0.5eq), slowly heat up to reflux and stir for 2 hours, HPLC central control: when the intermediate state content is less than 0.5%, and the temperature of the reaction solution drops to 15-25°C, pump Filter, transfer the filter cake to another 250mL reaction bottle, add a mixture of 60mL methanol and 60mL water for beating for 2 hours, filter with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com