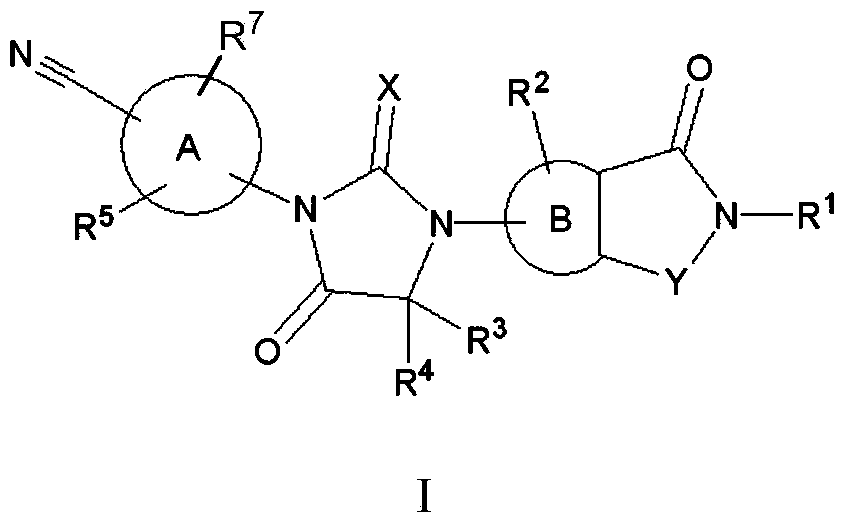
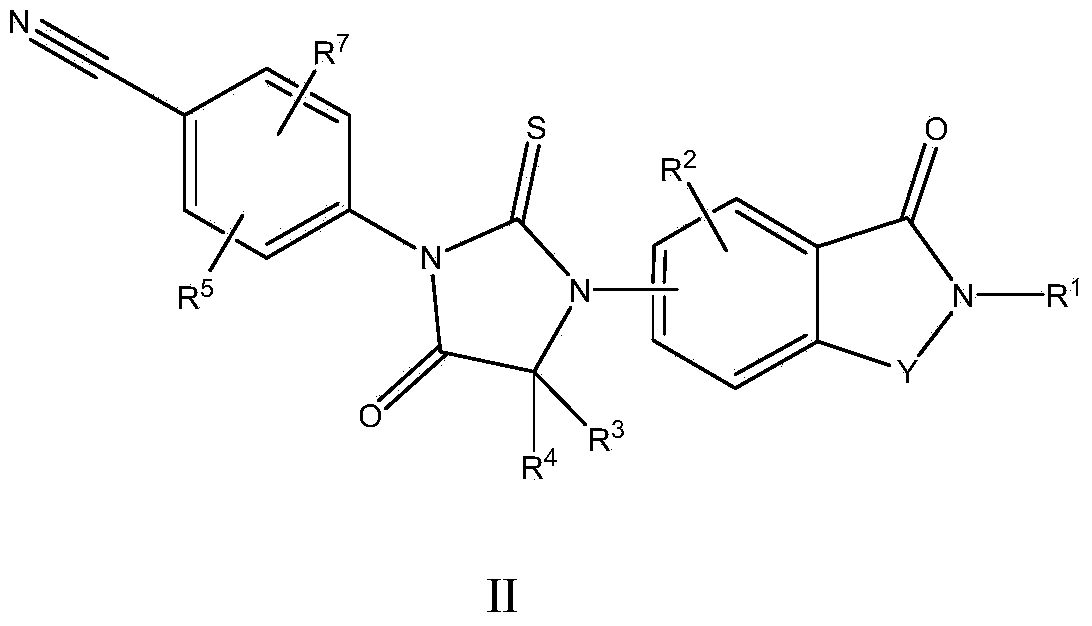
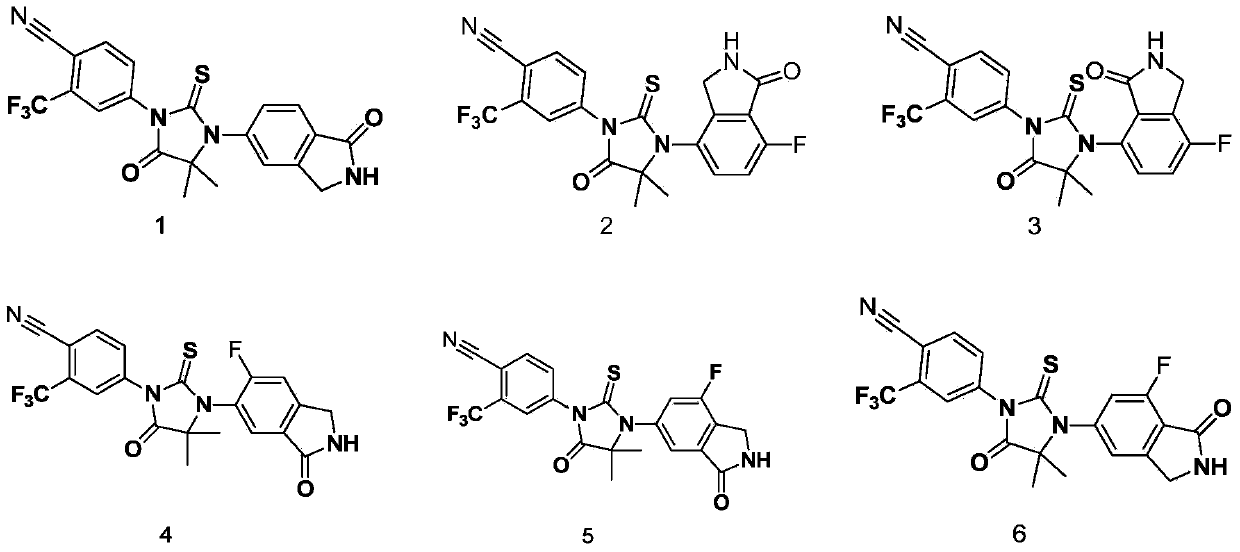
Biaryl hydantoin derivate and preparation method, medicine composition and application thereof
A technology of compounds and prodrugs, applied in the field of medicinal chemistry, can solve problems such as weak antagonistic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0112] Example 14-Isothiocyanato-2-trifluoromethylbenzonitrile (Intermediate 2)
[0113]
[0114]4-Amino-2-trifluoromethylbenzonitrile (10.0 g, 53.8 mmol) was added to n-hexane (22.5 mL) and water (25.0 mL), and the mixture was stirred at room temperature for 8 minutes after the addition. Under an ice bath, thiophosgene (4.5 mL, 58.5 mmol) was added dropwise to the above mixed system, and after the addition was completed, it was placed at room temperature and stirred overnight. Filter, wash the solid with n-hexane (2×25.0 mL), discard the solid, evaporate the filtrate to one-fifth of the original volume under reduced pressure, and place it in a refrigerator at 4°C overnight. After filtration, the solid was dried under high vacuum to obtain 11.0 g of white solid with a yield of 81%. 1 H NMR (CDCl 3 ,300MHz)δ(ppm)7.84(d,J=8.4Hz,1H),7.59(d,J=1.8Hz,1H),7.49(dd,J 1 =8.4Hz,J 2 =1.8Hz,1H).
Embodiment 2
[0115] Example 22-methyl-4-nitrobenzoic acid methyl ester
[0116]
[0117] In an ice bath, SOCl 2 (4.8mL, 66.2mmoL) was added dropwise into anhydrous methanol (100mL). Then, a solution of 2-methyl-4-nitrobenzoic acid (10.0 g, 55.2 mmol) in anhydrous methanol (70 mL) was added dropwise, and the addition was completed in 1 hour. The mixed system was reacted in an oil bath at 70°C for 7 hours, and the reaction was complete as detected by TLC. After cooling, the solvent was evaporated under reduced pressure. The residue was dissolved in ethyl acetate (100 mL), saturated sodium bicarbonate (100 mL) was added, the layers were separated, and the aqueous phase was extracted once with ethyl acetate (50 mL). The organic phases were combined, dried over anhydrous magnesium sulfate, filtered, the solvent was evaporated under reduced pressure, and dried under high vacuum to obtain 10.5 g of a white solid with a yield of 98%. 1 H NMR (CDCl 3 ,300MHz)δ(ppm)8.10-8.01(m,3H),3.95(s,3H)...
Embodiment 3
[0118] Example 32-(bromomethyl)-4-nitrobenzoic acid methyl ester
[0119]
[0120] Add 2-methyl-4-nitrobenzoic acid methyl ester (200mg, 1.0mmoL), benzoyl peroxide (48.5mg, 0.2mmoL), N-bromosuccinimide (213mg, 1.2mmoL) to CCl 4 (15mL), under the protection of Ar gas, heated and refluxed for 7 hours, cooled, washed with saturated sodium bicarbonate (3×20mL) and saturated brine (20mL) successively, evaporated the solvent under reduced pressure, and the residue was washed with a flash column Chromatographic separation, the eluent was PE:EA=20:1, and 124 mg of white solid was obtained with a yield of 44%. 1 H NMR (CDCl 3 ,300MHz)δ(ppm)8.34-8.01(m,3H),4.97(s,2H),4.00(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com