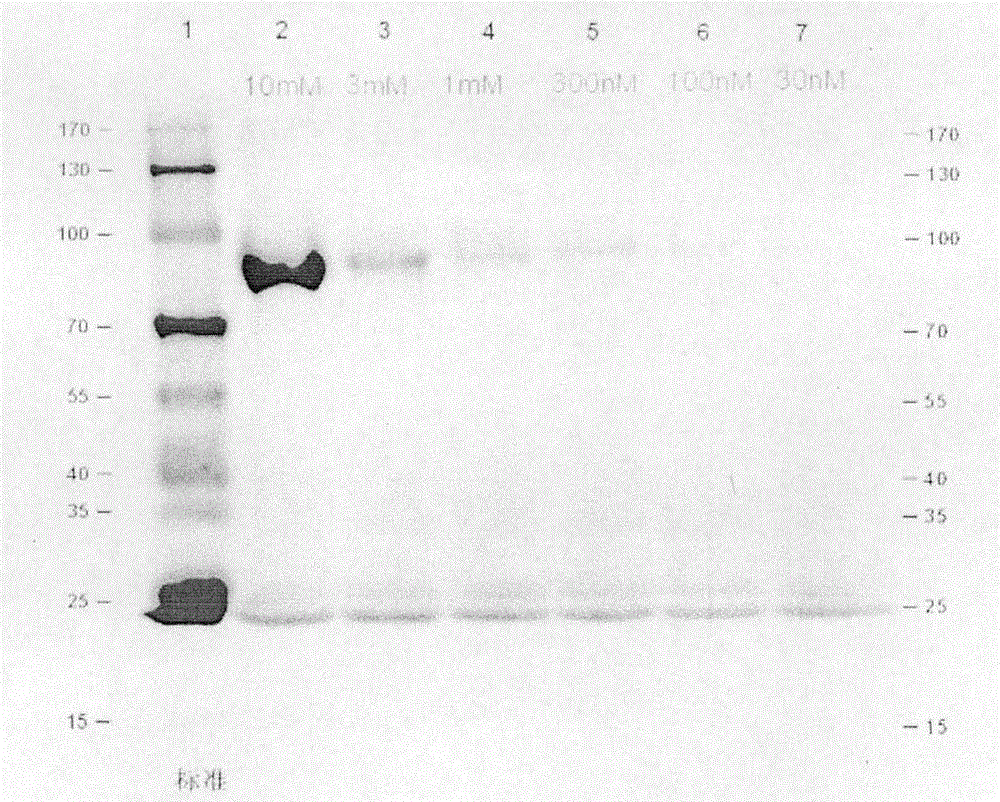
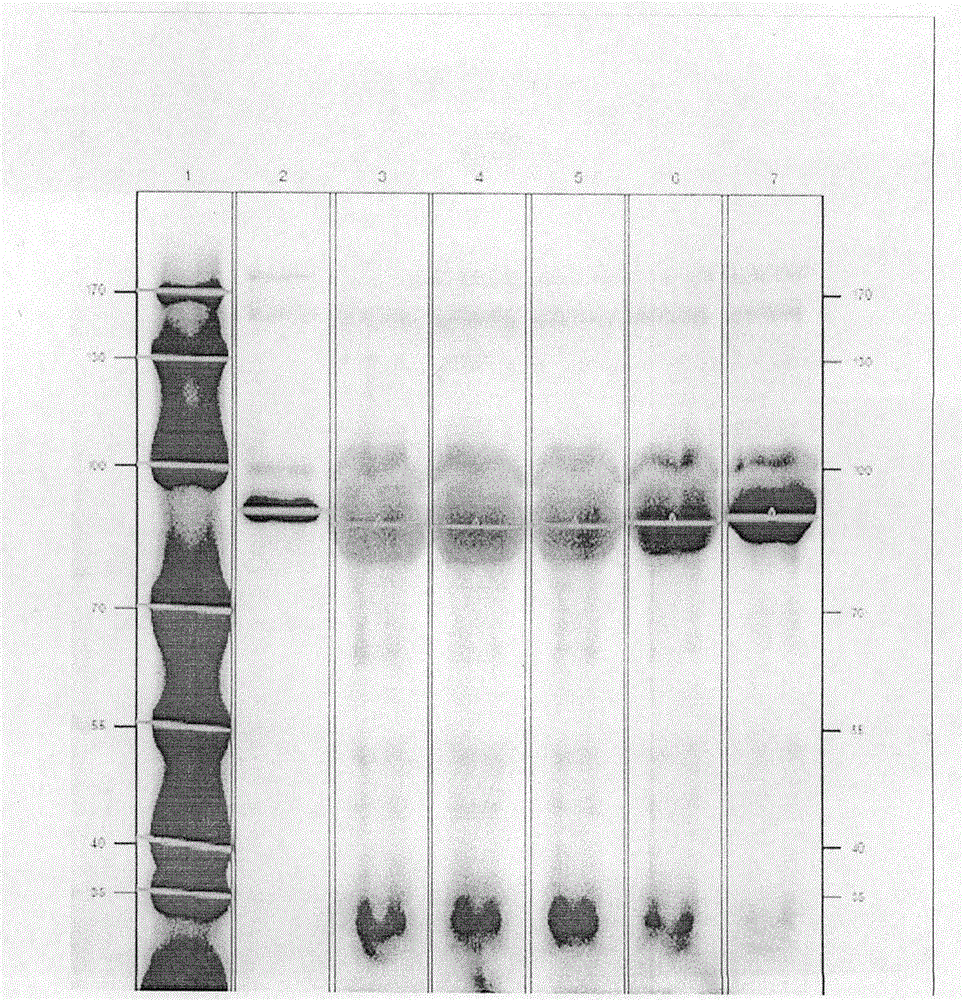
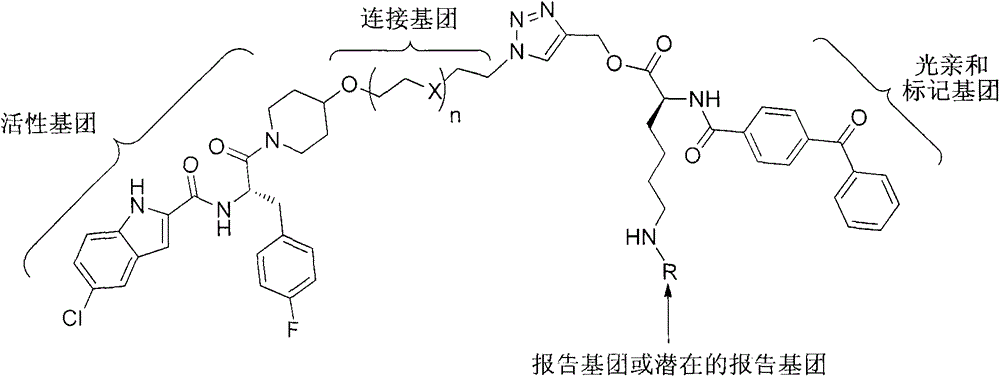
Photoaffinity labeling marker probe molecule for marking serum glycogen phosphorylase concentration level as well as preparation method and medical application of photoaffinity labeling marker probe molecule
A technology for probe molecules and labeled probes, which is applied in the field of photoaffinity-labeled probe molecules, and can solve problems such as temperature sensitivity and poor stability of antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]
[0043] 2-(2-{2-[2-(2-Methanesulfonyloxyethoxy)ethoxy]ethoxy}ethoxy)-tetrahydro-2H-pyran (2)
[0044] Tetraethylene glycol (8.90mL, 0.052mmol) and triethylamine (16.70mL, 0.12mol) were dissolved in 50mL of dichloromethane, and methanesulfonyl chloride (8.05mL, 0.104mol) was slowly added dropwise under cooling in an ice-water bath. After stirring at room temperature for 4h. The solvent was evaporated to dryness under reduced pressure, the residue was diluted with ethyl acetate and then washed with water, 1N hydrochloric acid, saturated NaHCO 3 solution and saturated brine, anhydrous Na 2 SO 4 After drying, the solvent was evaporated, and concentrated under reduced pressure to obtain 2-{2-[2-(2-methanesulfonyloxyethoxy)ethoxy]ethoxy}ethanol (8.70g, 62.1%), the product did not need further Purification was used directly in the next reaction. The above 2-{2-[2-(2-methanesulfonyloxyethoxy)ethoxy]ethoxy}ethanol (8.70 g, 31.97 mmol) was dissolved in anhydrous dichlorom...
Embodiment 2
[0091]
[0092] (S)-2-propynyl 6-(2-chloroacetylamino)-2-(4-benzoylbenzamido)hexanoate (14)
[0093] The above crude (S)-6-amino-2-(4-benzoylbenzamido)hexanoic acid-2-endynyl ester (5.00 g, 12.75 mmol) was dissolved in dry dichloromethane (300 mL) , added triethylamine (12.44 mL, 89.25 mmol), cooled to 0°C. Under ice-cooling, chloroacetyl chloride (5.76 mL, 76.49 mmol) was slowly added dropwise to the solution within 30 min, and then stirred at room temperature for 12 hours. The solvent was evaporated under reduced pressure, and the residue was diluted with dichloromethane, washed successively with saturated sodium bicarbonate solution and saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated. Flash column chromatography (petroleum ether / ethyl acetate: 8 / 1, V / V) gave a white solid (5.10 g, 86%).
[0094] ESI-MS m / z: 491.1 (M+Na) + .
[0095] 1 H-NMR (400MHz, CDCl 3 ): 1.49-1.66(m, 4H), 1.85-2.05(m, 2H), 2.54(t, J=2.4Hz, 1H), 3.32-3.38(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com