HPV16, 18L1 recombinant DNA vaccine for preventing and treating esophagus cancers
A HPV16, gene technology, applied in the field of tumor DNA vaccine preparation, can solve the problem that the safety cannot be guaranteed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 recombinant VR-HPV16, the construction of 18L1 DNA plasmid
[0025] 1.1 PCR amplification of HPV16, 18L1-pVR gene fragment
[0026] 1.1.1 The HPV16, 18L1-pVR gene fragment was amplified by PCR using pMK-RQ containing the codon-optimized HPV18L1 gene as a template and the synthetic sequence as a primer. After the completion of PCR, 1 μL of the reaction solution was taken for DNA gel (1%) electrophoresis detection. The PCR product was recovered after a good detection result was obtained.
[0027]1.1.2 Construction of HPV16L1 gene sequence: PCR amplification was carried out with the PUC-HPV16L1 plasmid containing HPV16L1 gene as template and the synthetic sequence as primer. dNTP8μl, Pfu2μl, ddH2O77μl, total system 100μl. After 30 cycles (denaturation at 94°C for 30s, annealing at 58°C for 30s, and extension at 72°C for 30s), the HPV16L1 gene sequence was obtained, and the PCR reaction product was purified by gel cutting.
[0028] 1.2 Ligation of HPV18L1-p...
Embodiment 2
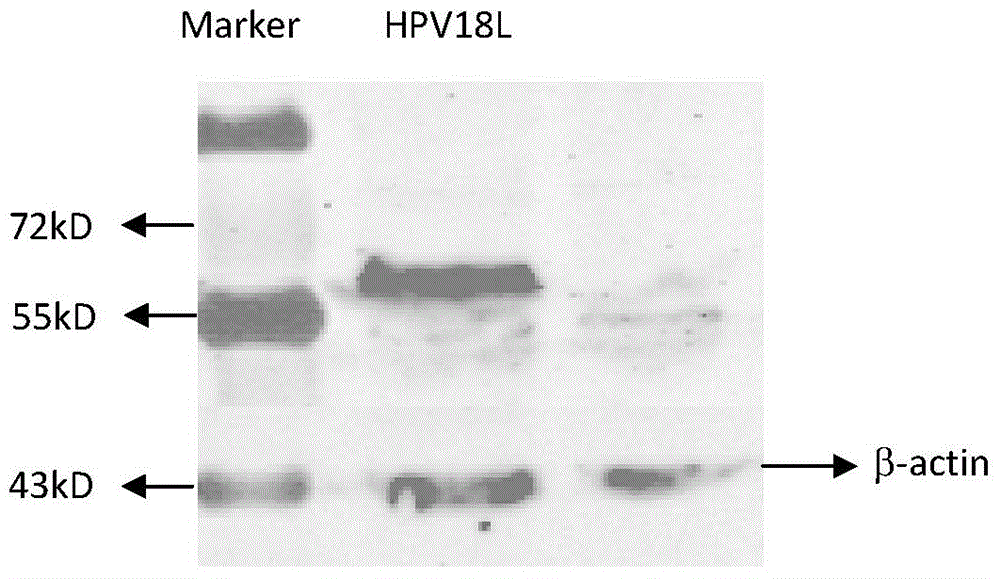
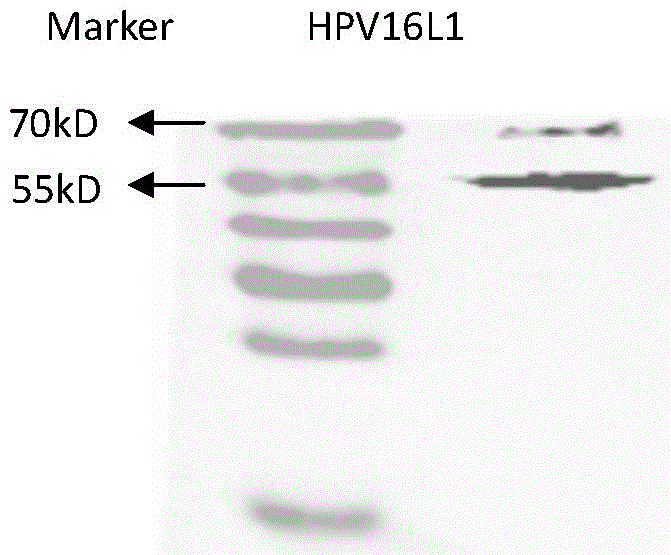
[0033] Example 2 Detection of pVR-HPV16, 18L1 expression in vitro
[0034] 2.1 Culture of HEK293 cells The medium used for culturing HEK293 cells is DMEM medium containing 10% FBS and 1% double antibody, and the culture conditions are 37°C, 5% CO 2 Constant temperature incubator.
[0035] Transfection of cells with the plasmid mentioned in 2.2
[0036] (1) The day before transfection, 293 cells were trypsinized and counted, transferred to a six-well plate, and the density was controlled so that the density on the day of transfection was close to 90%. Cells were plated in 2 mL of serum-containing normal growth medium without antibiotics.
[0037] (2) For each well of cells, dilute 4 μg of plasmid (pVR-HPV16, 18L1) with 250 μL of OPTI-MEM I medium. Mix Lipofectamine2000 reagent before use, and dilute 10 μL Lipofectamine2000 with 250 μL OPTI-MEM I medium. Mix gently, let stand at room temperature for 5 minutes, then mix with diluted plasmid, and let stand at room temperature ...
Embodiment 3
[0042] Example 3 Experiment of Recombinant DNA Vaccine pVR-HPV16, 18L1 Immune Mice
[0043] 3.1 Preparation and immunization plan for immunized mice
[0044] Twenty healthy female BALB / cH-2Kd mice of SPF grade 4-6 weeks old obtained from commercial sources were randomly divided into 3 groups, 10 mice in each group, and immunized twice at the 0th and 3rd week by intramuscular injection into the thigh. The immune response was measured one week after the booster immunization. See Table 1 for specific immunization contents and doses.
[0045] Table 1 Mouse immunization groups and doses
[0046]
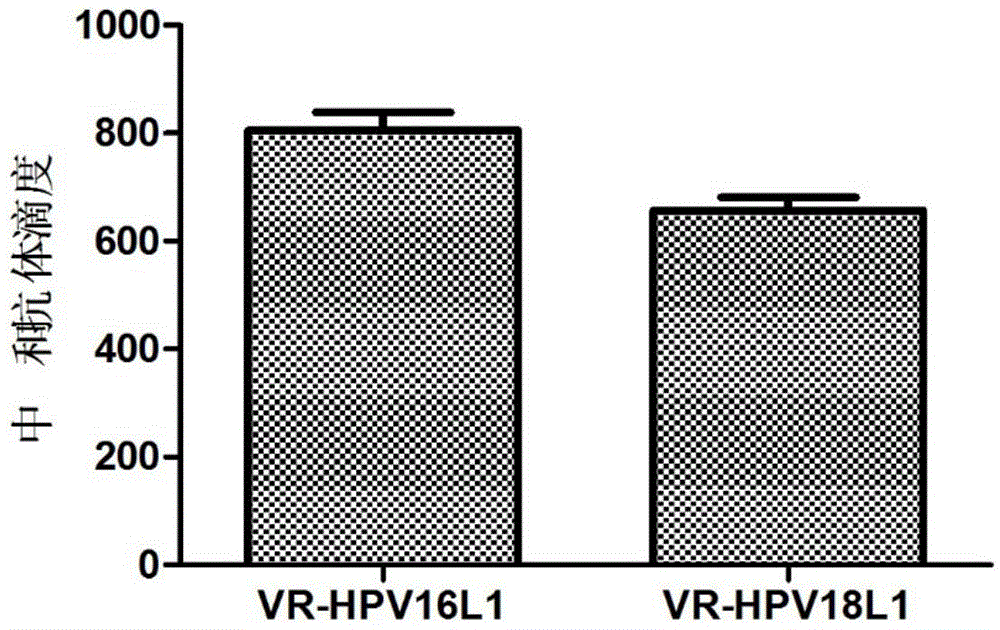
[0047] 3.2 Detection of HPV16, 18L1 humoral immune response in immunized mice
[0048] One week after the last booster immunization (day 28), the venous blood of the mice was collected, and the serum was separated. HPV16 and 18 pseudovirions were prepared according to the method reported in the literature (Buck, C.B., D.V. Pastrana, D.R. Lowy, et, al. J. Virol. 2004, 78:751-757.). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com