Method for preparing hydrogen CO with concentration by reforming methyl alcohol and biomass derivative in photocatalysis manner
A technology of biomass and derivatives, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve problems such as high content, limited hydrogen production activity, and no CO selectivity issues involved. Achieve the effect of improving hydrogen production activity and inhibiting CO production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] High temperature roasting method TiO 2 Sample preparation:
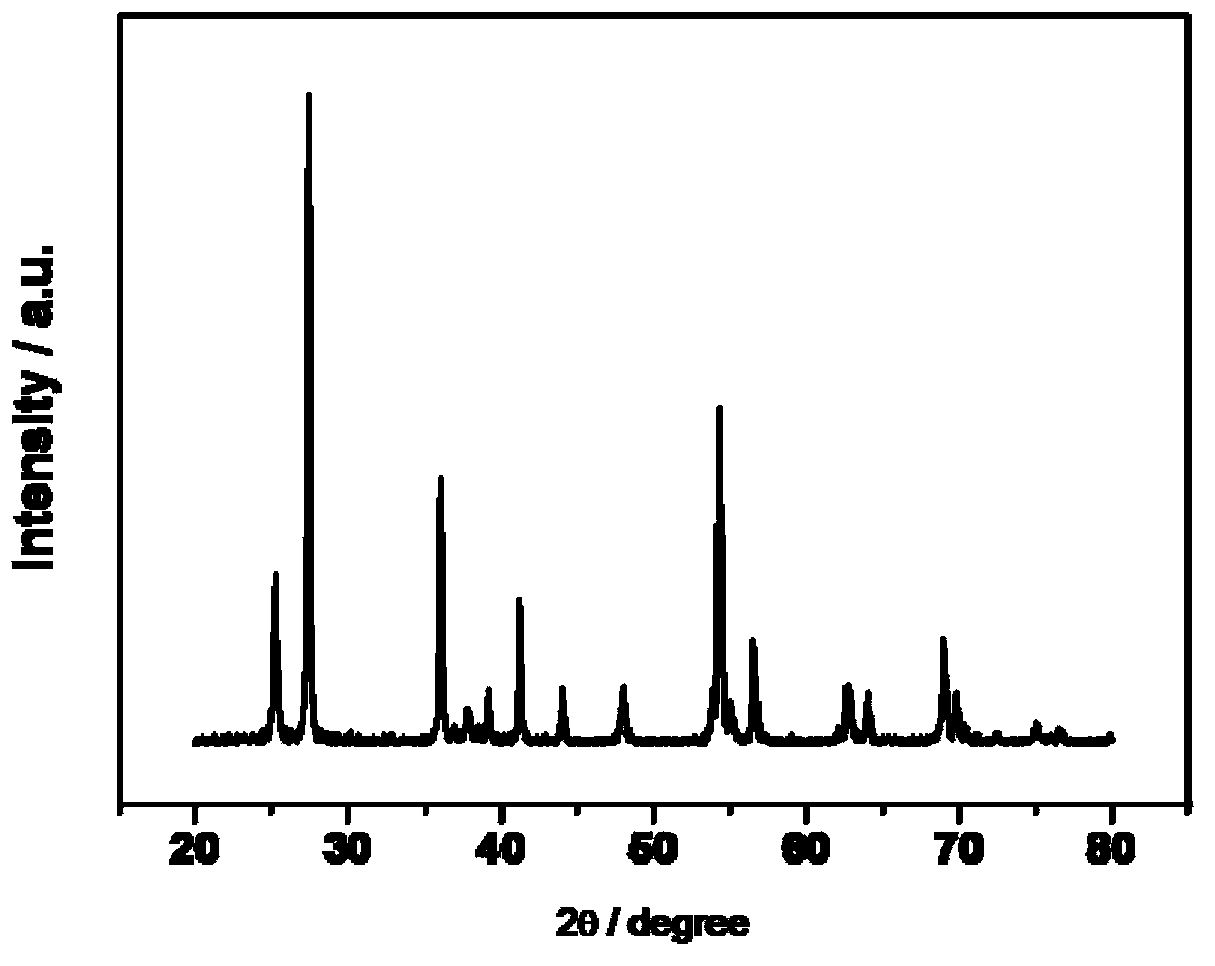
[0034] Weigh 1.0g of commercial P25 titanium oxide, roast it in a muffle furnace at 700°C in an air atmosphere for 3h, increase the temperature at a rate of 5°C / min, and drop it to room temperature naturally to obtain catalyst Cat 0, figure 1 Its XRD diffraction pattern.
Embodiment 2
[0036] Preparation of two-component cocatalysts by in situ photodeposition and evaluation of their photocatalytic reactions
[0037] In 200mL, 20vol% methanol solution, add 0.1g Cat 0 and a metal precursor with a single-component metal content of 0.1wt%. The amount added is: 0.169mL PdCl 2 solution (0.59mg / mL), and 0.138mL Ni(NO 3 ) 2 solution (0.725mg / mL), 0.136mL AgNO 3 solution (0.737mg / mL), 0.435mL HAuCl 4 solution (0.23mg / mL), 0.521mL RhCl 3 solution (0.192mg / mL), 0.170mL RuCl 3 solution (0.588mg / mL) and 0.366mL Na 2 IrCl 6 One of the solutions (0.273mg / mL). After the air in the system was evacuated, a 300W xenon lamp was used as the light source for photocatalytic reaction. Among them, the cocatalyst is deposited on Cat 0 within a few minutes in the initial stage of the reaction to obtain catalysts Cat1~7, and the corresponding cocatalyst components are: Cat 1, Pd; Cat 2, Pd-Ni; Cat 3, Pd-Ni Ag; Cat 4, Pd-Au; Cat 5, Pd-Rh; Cat 6, Pd-Ru; Cat 7, Pd-Ir. The gas p...
Embodiment 3
[0039] Preparation of two-component cocatalyst by impregnation method
[0040] Weigh 0.1g Cat 0, add a metal precursor with a single-component metal content of 0.1wt% and an appropriate amount of deionized water once. (The amount of metal precursor added is: 0.169mL PdCl 2 solution (0.59mg / mL); or 0.169mL PdCl 2 solution (0.59mg / mL), and 0.138mL Ni(NO 3 ) 2 solution (0.725mg / mL), 0.136mL AgNO 3 solution (0.737mg / mL), 0.435mL HAuCl 4 solution (0.23mg / mL), 0.521mL RhCl 3 solution (0.192mg / mL), 0.170mL RuCl 3 solution (0.588mg / mL) and 0.366mL Na 2 IrCl 6 Solution (0.273mg / mL) impregnated and stirred for 3h, the solution was evaporated to dryness in a water bath at 80°C, after grinding, the catalyst precursor was placed in a tube furnace under a hydrogen atmosphere and roasted at 350°C for 2h (100% hydrogen, flow rate 100mL / min), the catalyst Cat 1'-7' was obtained. The corresponding metal components are: Cat 1', Pd; Cat 2', Pd-Ni; Cat 3', Pd-Ag; Cat 4', Pd-Au; Cat 5', P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com