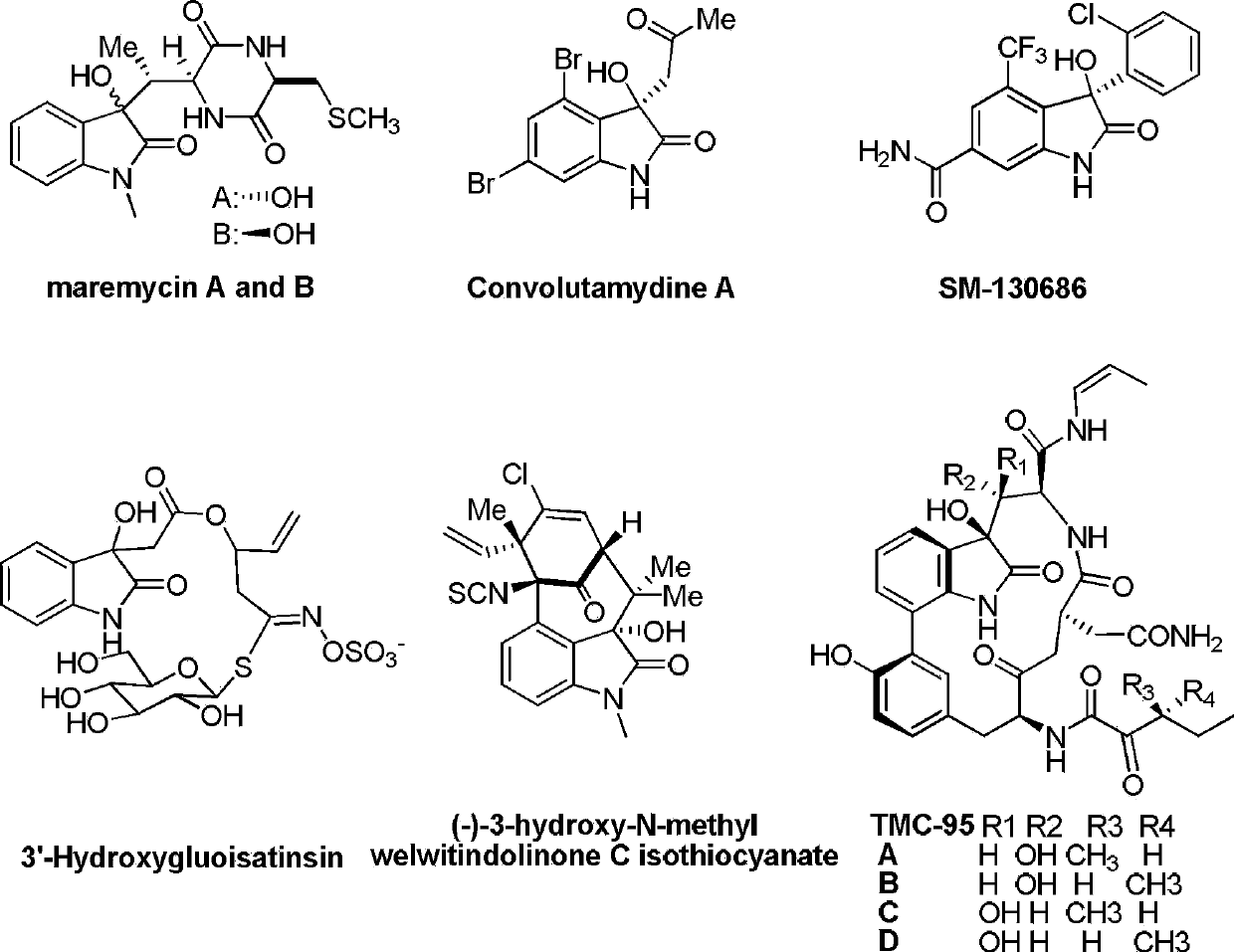
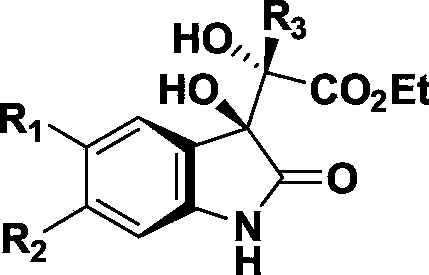
Preparing method of 3-hydroxyloxoindole derivatives
A technology for indole derivatives and hydroxyl oxidation, applied in the direction of organic chemistry, can solve the problems of complex product purification process, unfavorable industrial application, long reaction cycle, etc., and achieve the effect of shortening the production cycle, avoiding column chromatography, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] First weigh isatin (1mmol): copper sulfate = 1:0.01 (molar ratio), place it in a 50mL round bottom flask, add 10mL of water to make a mixed solution of isatin and copper sulfate; then, add ethyl diazoacetate (2mmol) mixed with another 10mL of water evenly, then, inject the diazo solution into the mixed solution of isatin and copper sulfate by a peristaltic pump for 1 hour, stir at room temperature for 1-2 hours, and extract with 30mL of petroleum ether to remove the small electrode After extraction with 30 mL of ethyl acetate three times (10 mL / time), the organic layers were combined, washed with 30 mL of saturated brine, dried with anhydrous sodium sulfate for 3 hours, and then 40 ° C rotary evaporation to remove ethyl acetate, the crude product was obtained by volume The pure product of 3-hydroxyoxindole derivative 1a was obtained by recrystallization from petroleum ether and ethyl acetate at a ratio of 1:1. Yield: 90%, dr value: 80:20. 1 H NMR (400MHz, DMSO-d 6 )...
Embodiment 2
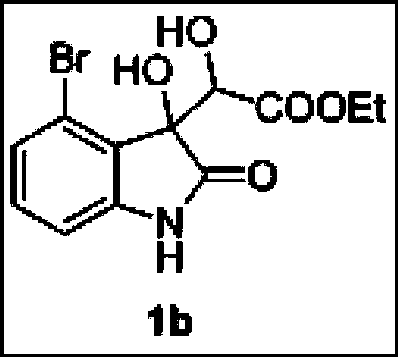
[0026] First weigh 4-bromoisatin (1mmol):rhodium acetate=2:0.01 (molar ratio), place in a 50mL round bottom flask, add 10mL tetrahydrofuran (water) to make isatin and rhodium acetate mixed solution; then, Ethyl diazoacetate (2mmol) was mixed uniformly with another part of 10mL tetrahydrofuran (water), and then, the diazo solution was injected into the mixed solution of isatin and rhodium acetate by a peristaltic pump for 1 hour, and after stirring at room temperature for 1-2 hours, Extract with 30mL of petroleum ether to remove small polar impurities, then extract with 30mL of ethyl acetate three times (10mL / time), combine the organic layers, wash with 30mL of saturated brine, dry with anhydrous sodium sulfate for 3 hours, and then remove by rotary evaporation at 40°C Ethyl acetate, the crude product was recrystallized from petroleum ether and ethyl acetate at a volume ratio of 1:1 to obtain the pure product of 3-hydroxyoxindole derivative 1b. Yield: 40%, dr value: 83:17.
[00...
Embodiment 3
[0030] First weigh 5-methyl isatin (1mmol): ruthenium chloride = 2:0.01 (molar ratio), put it in a 50mL round bottom flask, add 10mL 1,2-dichloroethane (water) to make isatin Mix solution with ruthenium chloride; Next, mix ethyl diazoacetate (2mmol) with another part of 10mL 1,2-dichloroethane (water), and then inject the diazo solution for 1 hour through a peristaltic pump In the mixed solution of isatin and ruthenium chloride, after stirring at room temperature for 1-2 hours, extract with 30mL petroleum ether to remove small polar impurities, and then extract with 30mL ethyl acetate three times (10mL / time), combine the organic layers, and 30mL saturated salt After washing with water, drying with anhydrous sodium sulfate for 3 hours and then rotary evaporation at 40°C to remove ethyl acetate, the crude product was recrystallized from petroleum ether and ethyl acetate at a volume ratio of 1:1 to obtain 3-hydroxyindole derivative 1c pure Taste. Yield: 85%, dr value: 89:11.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com