Fusion toxin with specificity of VEGFR2/KDR acceptor, and coding gene and application thereof
A technology encoding gene and receptor specificity is applied to fusion toxin with VEGFR2/KDR receptor specificity and its encoding gene and application field, which can solve the problems of normal tissue damage and poor specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
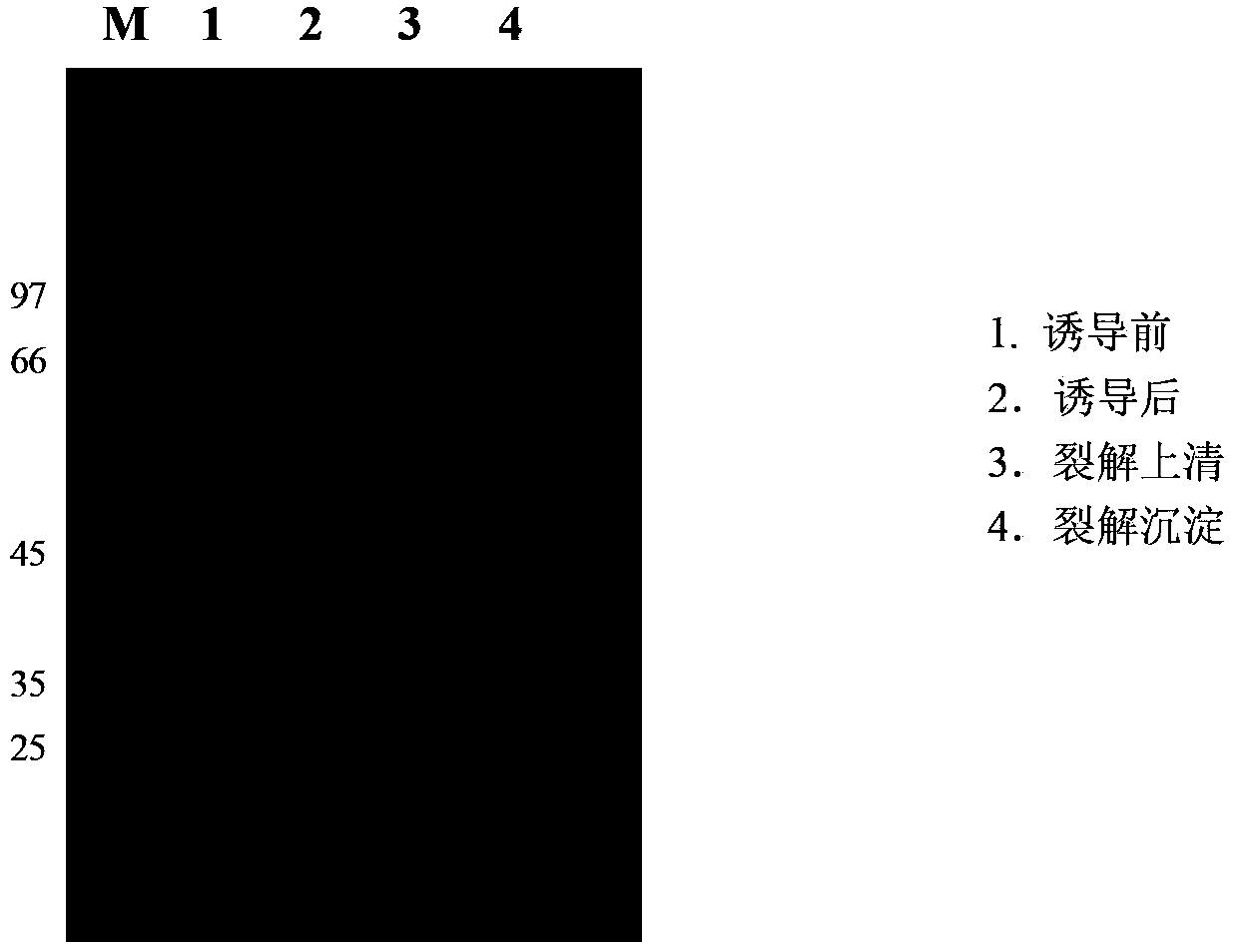
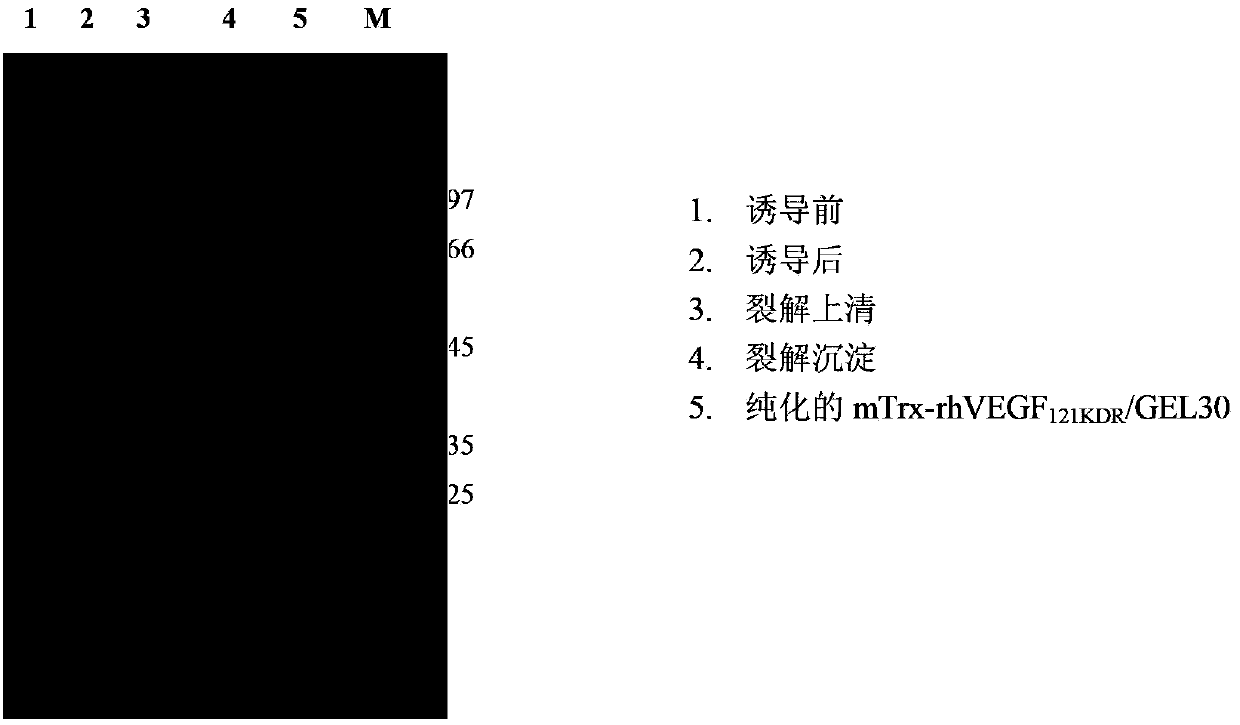
[0108] Embodiment 1, fusion toxin rhVEGF 121KDR Expression and purification of rGEL30
[0109] 1. Construction of recombinant expression vector pTrx-rhVEGF 121KDR / rGEL30 and pET32a(+)-rhVEGF 121KDR / rGEL30
[0110] 1. Construction of recombinant expression vector pTrx-rhVEGF 121KDR / rGEL30
[0111] 1) The amino-terminal (N-terminal)-linked thioredox protein (Trx) mutant (mTrx) was synthesized by Beijing Aoke Company (the amino acid residue sequence of the thioredox protein mutant mTrx is shown in SEQ ID NO: 7 in the sequence listing The nucleotide sequence of its coding gene is shown in SEQ ID NO: 8 in the sequence listing. SEQ ID NO: 7 in the sequence listing consists of 109 amino acid residues, starting from the 33rd-36th (4aa) amino acid at the amino terminal The residues are Cys-Pro-Tyr-Cys, the 34th amino acid residue from the amino terminal is a mutant amino acid residue Pro34, and the 35th amino acid residue from the amino terminal is a mutant amino acid residue T...
Embodiment 2
[0128] Example 2, rhVEGF 121KDR Toxicity detection of / rGEL30 fusion toxin on PAE / KDR and PAE / FLT cells
[0129] detect rhVEGF 121KDR The toxic effect of / rGEL30 fusion toxin on PAE / KDR and PAE / FLT cells, the specific method comprises the following steps:
[0130] 1) Dilute PAE / KDR and PAE / FLT cells in logarithmic growth phase to 1.5×10 4 / mL, add to 96-well plate, 200 μl per well (3000 cells / well) (no cells in the first row), 37°C, 5% CO 2 Incubate overnight. Gradients in 96-well plates (10 -3 、10 -2 、10 -1 、10 0 、10 1 、10 2 、10 3 、10 4 ) release rhVEGF 121KDR / rGEL30 fusion toxin (initial concentration 1 μM) in a final volume of 200 μl per well. Pour off the medium in the cell culture wells, add fusion toxins of different concentrations (add fresh medium to the first row, and not add toxin to the second row), at 37°C, 5% CO 2 Incubate for 72 hours.
[0131] 2) Pour off the medium in the cell culture well, add 100 μl 0.5% crystal violet solution (prepared with ...
Embodiment 4
[0135] Example 4, rhVEGF 121KDR / rGEL30 Fusion Toxin Animal Antitumor Pharmacodynamics Observation
[0136]Take 5-week-old Balb / c nude mice, female, weighing 18-20 g. The colon cancer HT-29 cell line was inoculated subcutaneously in the back of nude mice, and the tumor grew to about 200 (mm) 3 After that, take it out and homogenize, so that it can be passaged twice in vivo, and then in 1×10 6 / 0.2mL concentration and inoculated subcutaneously in the back of nude mice respectively, and sent them back to the animal breeding room to continue feeding after inoculation. About a week after inoculation, hard nodules with a diameter of 2-3 mm can be felt subcutaneously at the inoculation site of the mice, indicating that the mouse xenograft tumor model was established successfully. The animals were randomly divided into 4 groups, 6 animals in each group. Start medication.
[0137] Dosage, Frequency and Duration of Administration:
[0138] Negative control group (negative control...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com