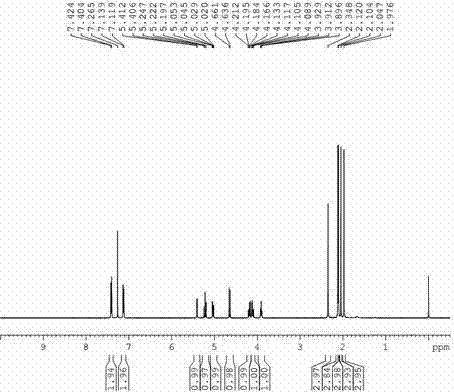
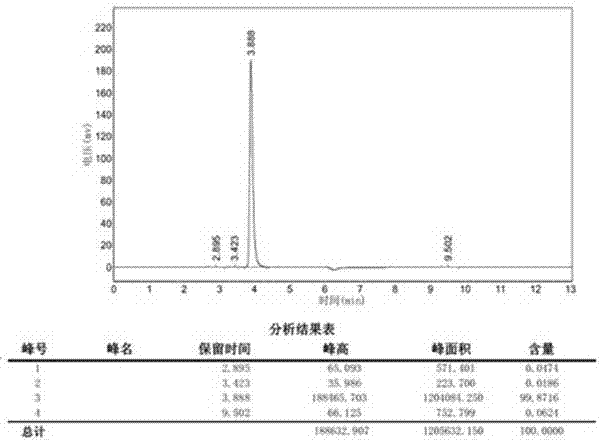
Preparation method of p-methylphenyl-beta-D-thioacetylgalactoside
A technology of thioacetyl and p-tolyl, which is applied in the field of sugar compound synthesis, can solve the problems of using unstable intermediates, numerous steps, complicated operations, etc., and achieve the effects of improving production safety factor, readily available raw materials, and simple operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 5.5:1:1:1.1:4.5
[0026] a) Add 5.5 mol of acetic anhydride and 1 mol of aluminum trichloride to the reactor at room temperature, add 1 mol of D-galactose at 50-55 °C under temperature control, and react for 2 hours. After the reaction is completed, add 1.1 mol of p-toluene into the reaction droplet In thiophenol and 4.5mol of dichloromethane solution, the temperature was controlled to react at 15-20 ° C for 2 hours, after completion, 0.904mol of p-tolyl-β-D-thioacetylgalactoside was obtained through post-treatment, and the yield was 90.4%, purity 99.78%.
[0027] In step a, post-processing is to add dropwise 10 mol of deionized water at 0-5°C and stir for 1 hour, extract the aqueous layer with 4 mol of dichloromethane, separate the organic phase and wash the organic phase three times with 8 mol of deionized water, and separate the organic phase. Concentrated, crystallized by adding a mixture of 1 mol tert-butyl methyl ether and 3 mol isohexane, filtered an...
Embodiment 2
[0028] Example 2 5.5:1.5:1:1.2:4.5
[0029] a) Add 5.5 mol of acetic anhydride and 1.5 mol of aluminum trichloride at room temperature, add 1 mol of D-galactose at 50-55 °C under temperature control, and react for 3 hours. After the reaction is completed, add 1.2 mol of p-toluenethiophenol and In 4.5 mol of dichloromethane solution, the temperature was controlled to react at 15-20 ° C for 3 hours, and after completion, 0.911 mol of p-tolyl-β-D-thioacetylgalactoside was obtained through post-treatment, and the yield was 91.1%, Purity 99.81%.
[0030] In step a, post-processing is to add dropwise 10 mol of deionized water at 0-5°C and stir for 1 hour, extract the aqueous layer with 4 mol of dichloromethane, separate the organic phase and wash the organic phase three times with 8 mol of deionized water, and separate the organic phase. Concentrated, crystallized by adding a mixture of 1 mol tert-butyl methyl ether and 3 mol isohexane, filtered and dried.
Embodiment 3
[0031] Example 3 6:1.5:1:1.3:5.0
[0032] a) Add 6 mol of acetic anhydride and 1.5 mol of aluminum trichloride at room temperature, add 1 mol of D-galactose at 50-55 °C under temperature control, and react for 2.5 hours. After the reaction is completed, add 1.3 mol of p-toluenethiophenol and 5.0 mol of dichloromethane solution, the temperature was controlled to react at 15-20 ° C for 4 hours, and after completion, 0.907 mol of p-tolyl-β-D-thioacetylgalactoside was obtained through post-treatment, with a yield of 90.7% and a purity of 90.7%. 99.76%.
[0033] In step a, the post-treatment is to add dropwise 8 mol of deionized water at 0-5°C and stir for 1 hour, extract the aqueous layer with 4 mol of dichloromethane, separate the organic phase and wash the organic phase three times with 8 mol of deionized water, and separate the organic phase. Concentrated, crystallized by adding a mixture of 1 mol tert-butyl methyl ether and 2 mol isohexane, filtered and dried.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com