Method for preparing crosslinked NAMPT (Nicotinamide Phosphoribosyltransferase) and screening NAMPT inhibitor
An inhibitor, the technology of group A, applied in the direction of biochemical equipment and methods, microbiological measurement/testing, enzymes, etc., can solve the problems of low work efficiency, many reaction steps, complicated operation, etc., and achieve high work efficiency and reflection steps The effect of less and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The method for preparing cross-linked NAMPT and screening NAMPT inhibitors of the present invention comprises the following steps:
[0052] S1: Preparation of NAMPT recombinant protein.
[0053] S2: Add 1 part of NAMPT recombinant protein to 20-100 parts (preferably 30 parts) of reducing agent according to molar parts. The reducing agent can be selected from DTT (dithiothreitol) or TCEP (tris(2-carboxyethyl) ); incubate at a temperature of 20°C to 25°C for 0.5h to 1.5h (preferably 1h) to obtain crude protein, and use a desalting column to remove the reducing agent in the crude protein to obtain pretreated protein;
[0054] S3: Add 1 part of pretreated protein and 2 to 5 parts of cross-linking reagent BMB (1,4-bis(maleimido)butane) into the reaction kettle according to the molar parts, mix well, and put them at a temperature of 20℃~ After reacting for 1h-5h (preferably 2h) at 25°C, use a desalting column to remove unreacted BMB to obtain cross-linked NAMPT;
[0055] S4...
Embodiment 1
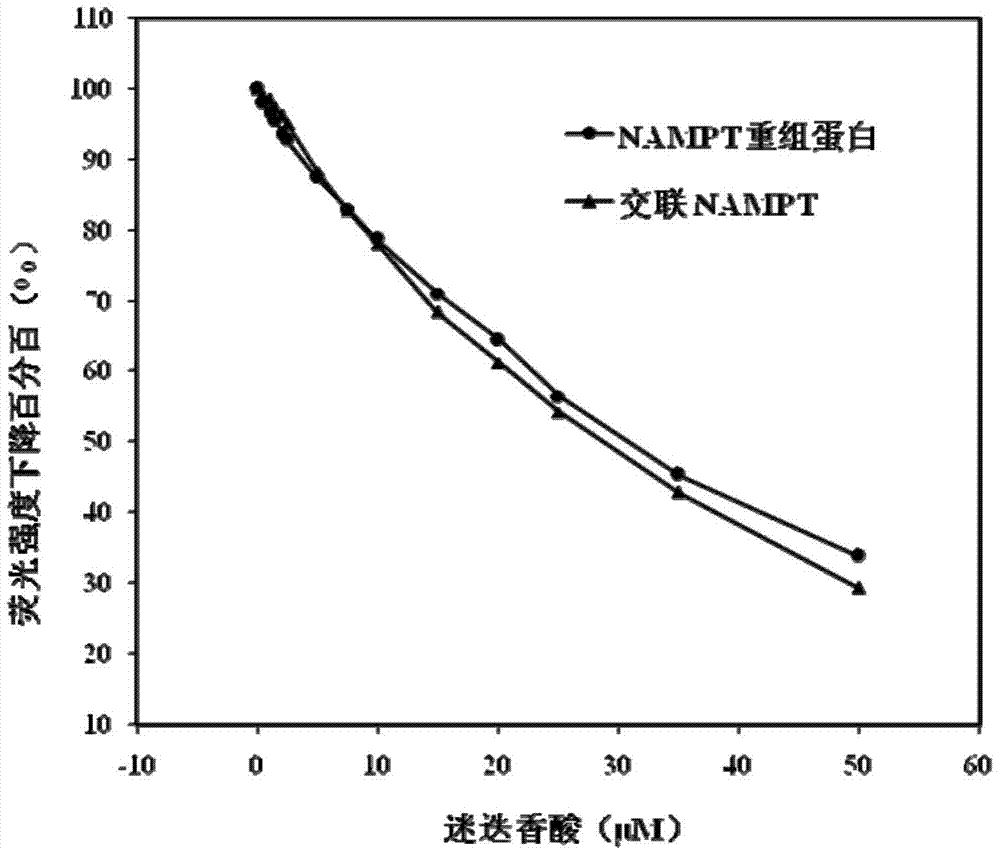
[0075] Example 1, the candidate inhibitor is selected as rosmarinic acid.
[0076] S1: Preparation of NAMPT recombinant protein.
[0077] S2: Add 2 mL of NAMPT recombinant protein with a concentration of 200 μM to 1.6 mL of DTT (dithiothreitol) with a concentration of 5 mM, and incubate for 1 h at a temperature of 20 ° C to obtain crude protein, and use a desalting column to remove the crude protein In the DTT, the pretreated protein was obtained.
[0078] S3: Add the pretreated protein and 80 μL of BMB cross-linking reagent with a concentration of 10 mM into the test tube, mix well, react at 20° C. for 2 hours, and remove excess BMB with a desalting column to obtain cross-linked NAMPT.
[0079] S4: preset PBS buffer solution, divide the PBS buffer solution into three parts: the first PBS buffer solution, the second PBS buffer solution and the third PBS buffer solution, wherein the volume of the first PBS buffer solution and the second PBS buffer solution Similarly, NAMPT re...
Embodiment 2
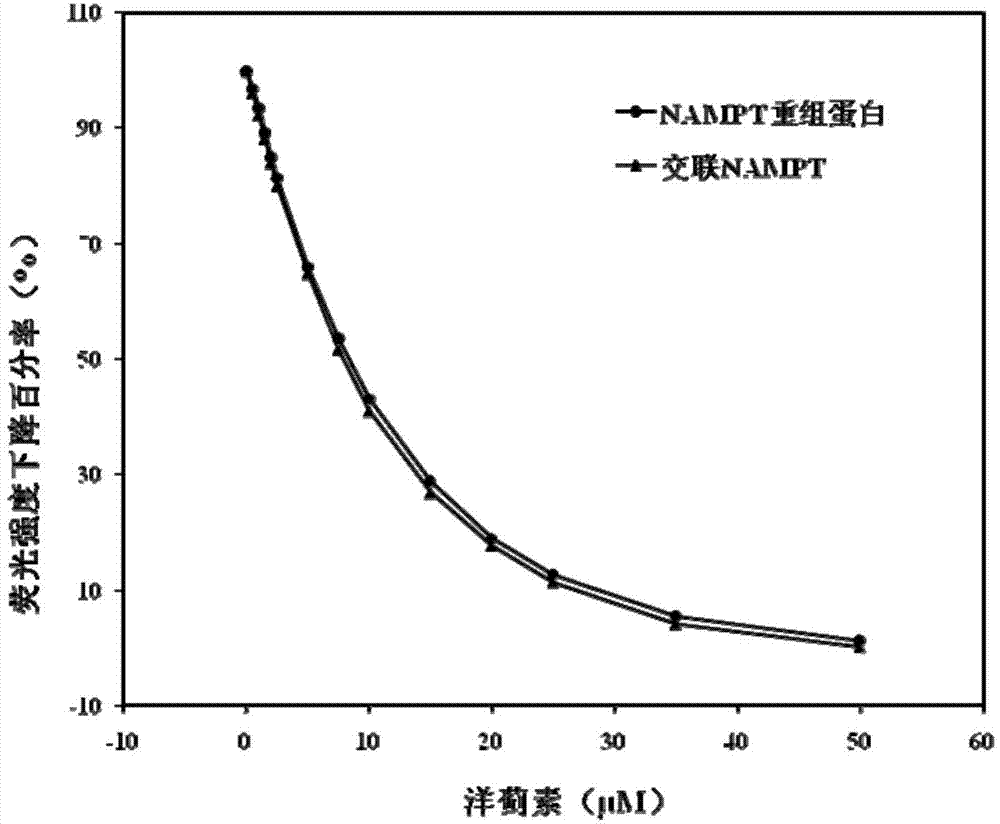
[0090] Example 2: Selection of the candidate inhibitor as cynarin.
[0091] S1: Preparation of NAMPT recombinant protein.
[0092] S2: Add 3 mL of NAMPT recombinant protein with a concentration of 200 μM to 36 mL of ECET with a concentration of 1 mM, incubate for 0.5 h at a temperature of 25°C to obtain crude protein, and use a desalting column to remove DTT in the crude protein to obtain pretreated protein .
[0093] S3: Add the pretreated protein and 240 μL of BMB with a concentration of 10 mM into the test tube, mix well, react at a temperature of 20° C. for 1 hour, and remove excess BMB with a desalting column to obtain cross-linked NAMPT.
[0094] S4: preset PBS buffer solution, divide the PBS buffer solution into three parts: the first PBS buffer solution, the second PBS buffer solution and the third PBS buffer solution, wherein the volume of the first PBS buffer solution and the second PBS buffer solution Similarly, NAMPT recombinant protein was added to the first PBS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com