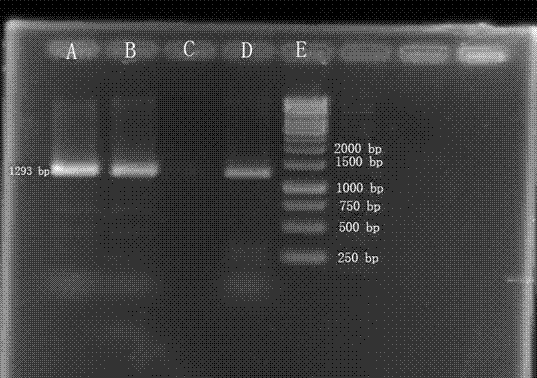
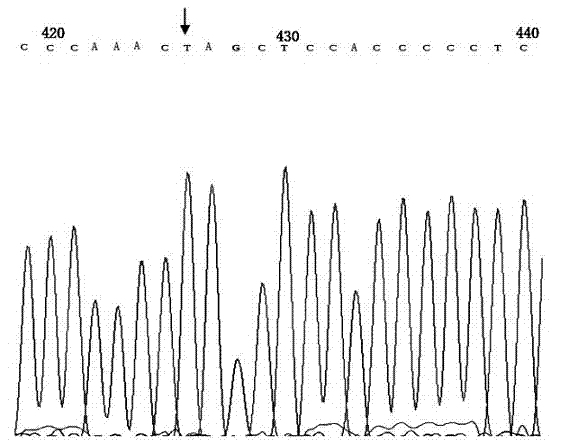
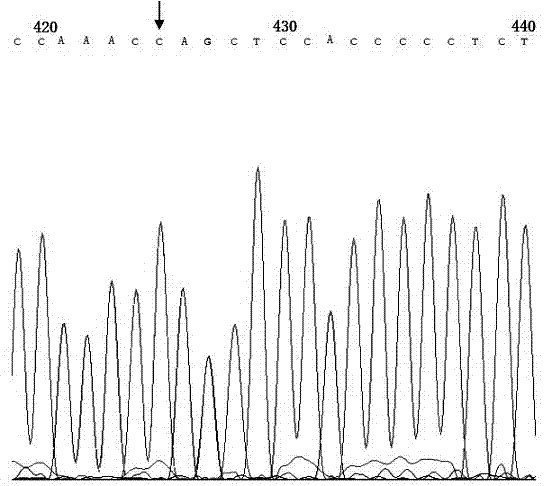
Genetic diagnosis kit for amyotrophic lateral sclerosis
A technology for lateral sclerosis and genetic diagnosis, applied in the field of molecular biology, can solve the problems of patents and reports of molecular diagnosis of ALS diseases that have not yet been seen, and achieve great popularization and application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Gene determination, primer design, kit composition and amplification procedure
[0039] The 11 genes involved in the present invention are related to ALS disease, and the correlation has been reported in literature. The sequences of 11 genes can be obtained through the following ways.
[0040] The gene sequence can be retrieved at http: / / www.ncbi.nlm.nih.gov / according to the following gene ID
[0041] Table 2 Gene ID
[0042]
[0043] In the process of primer design, it is mainly designed according to the conserved region of each gene, and the entire CDS region of each gene is specifically amplified by using primers. The forward and reverse primers are respectively designed in the 5'-UTR and 3'-UTR regions to ensure that the amplified product can cover all CDS regions, enabling the kit to detect mutation sites on all CDS regions.
[0044] Taking the PFN1 gene as an example, the gene sequence obtained after downloading is shown below (SEQ ID NO.23), wh...
Embodiment 2
[0057] Example 2 Detection of genetic diagnosis of amyotrophic lateral sclerosis
[0058] The gene diagnosis kit for familial amyotrophic lateral sclerosis (ALS) provided by the present invention can detect samples such as blood, body fluid, hair, bone, cell or tissue samples of the individual to be tested. In this example, the same standard cell line as in Example 1 was used.
[0059] 1. Extraction of RNA from individual human fibroblast skin cells to be tested:
[0060] 1. Take the sample to be tested, add 1ml Trizol solution, mix on ice, mash and homogenate; stand on ice for 10min;
[0061] 2. Add 200μl chloroform to the homogenate, shake vigorously for 15s, and incubate at room temperature for 10min
[0062] 3. Centrifuge at 12000g / min at 4°C for 15min
[0063] 4. Take the aqueous phase (upper layer), add 500μl isopropanol, shake gently, and incubate at room temperature for 10~15min
[0064] 5. 4°C, 12000g / min, l centrifuge for 10min
[0065] 6. Discard the supernatan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com