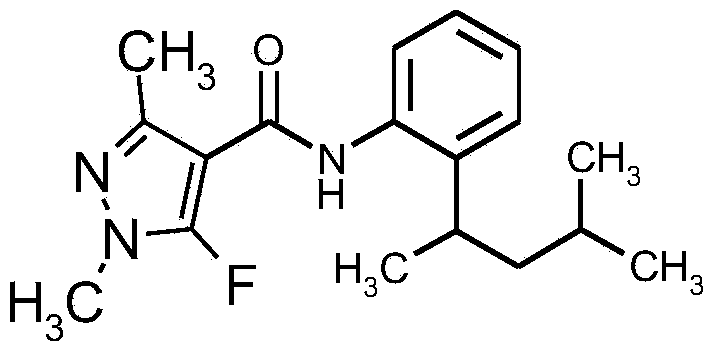
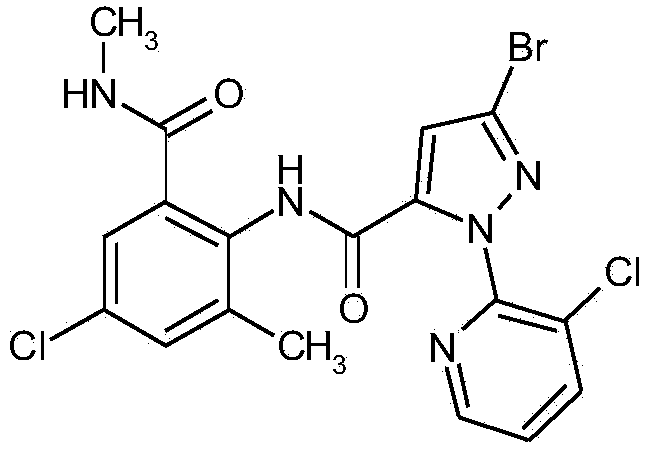
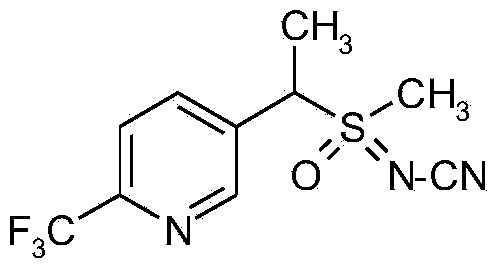
Synergistic active ingredient combinations containing penflufen
A technology of flufenapyridine and conjugates, which is applied in the field of new active compound conjugates, and can solve problems that have not been specifically disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0302] Solanum Rhizoctonia test (in vitro) / microtiter plate
[0303] Potato dextrose broth (PDB) was used as the liquid test medium in the microtiter plate for micro test. The active compound is used as an industrial grade active ingredient dissolved in acetone. A suspension of mycelium of Rhizoctonia solani is used for inoculation. After inoculating in the dark and shaking (10 Hz) for 7 days, the transparency of each filled cavity of the microtiter plate was measured by a spectrophotometer.
[0304] Here, 0% means the efficacy corresponding to the growth condition of the control group, and the efficacy of 100% means that no fungal growth is observed.
[0305] The following table clearly shows that the measured activity of the active compound combination of the present invention is greater than the calculated activity, that is, there is a synergistic effect.
[0306] form
[0307] Solanum Rhizoctonia test (in vitro) / micro test
[0308]
[0309]
Embodiment 2
[0311] Sclerotium rotundus test (in vitro) / microtiter plate
[0312] Potato dextrose broth (PDB) was used as the liquid test medium in the microtiter plate for micro test. The active compound is used as an industrial grade active ingredient dissolved in methanol. A spore suspension of P. rotundus was used for inoculation. After inoculation in the dark and shaking (10 Hz) for 5 days, the transparency of each filled cavity of the microtiter plate was measured by means of a spectrophotometer.
[0313] Here, 0% means the efficacy corresponding to the growth condition of the control group, and the efficacy of 100% means that no fungal growth is observed.
[0314] The following table clearly shows that the measured activity of the active compound combination of the present invention is greater than the calculated activity, that is, it exists in a synergistic effect.
[0315] form
[0316] Sclerotium rotundus test (in vitro) / micro test
[0317]
[0318]
Embodiment 3
[0320] The larvae test of horseradish beetle
[0321] Solvent: 7 parts by weight of dimethylformamide
[0322] Emulsifier: 2 parts by weight of alkyl aryl polyglycol ether
[0323] To prepare a suitable active compound preparation, 1 part by weight of the active compound is mixed with the stated amount of solvent and emulsifier, and the concentrate is diluted with water containing the emulsifier to the desired concentration.
[0324] Chinese cabbage leaves (Brassica oleracea) are treated by spraying with a preparation of active compound of the desired concentration, and inoculated with larvae of the horseradish leaf beetle (Hormania oleracea) while the leaves are still moist.
[0325] After the required period of time, the kill rate is determined in %. Here, 100% means that all beetle larvae have been killed; 0% means that no beetle larvae have been killed. The determined kill rate was calculated using Colby's formula.
[0326] In this test, the following active compound combinations...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com