Hydronopyl triethyl ammonium iodide preparation method and application
A technology of hydrogenated nopyl triethyl ammonium iodide and hydrogenated nopyl iodoalkane, applied in the field of medicine, can solve the problems of no inhibition of tumor cell growth, no anti-cancer activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
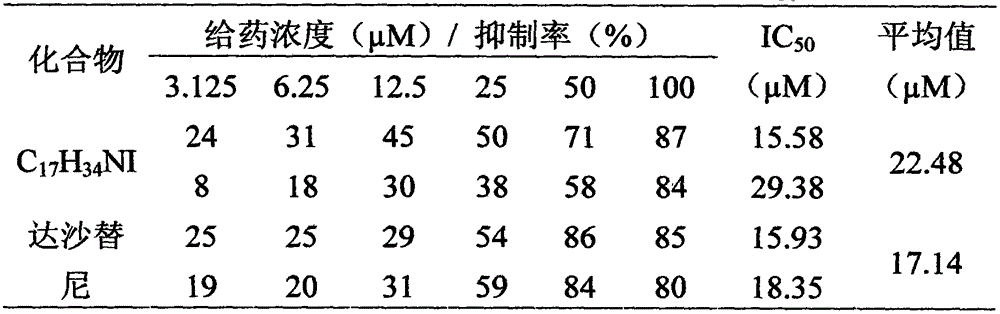
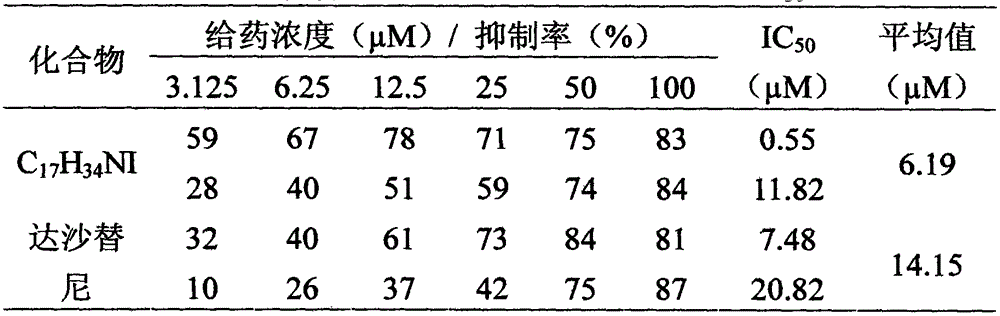
[0011] Below in conjunction with the examples, the specific implementation of the present invention will be further described in detail. The following examples are used to illustrate the present invention, but are not intended to limit the scope of the present invention.
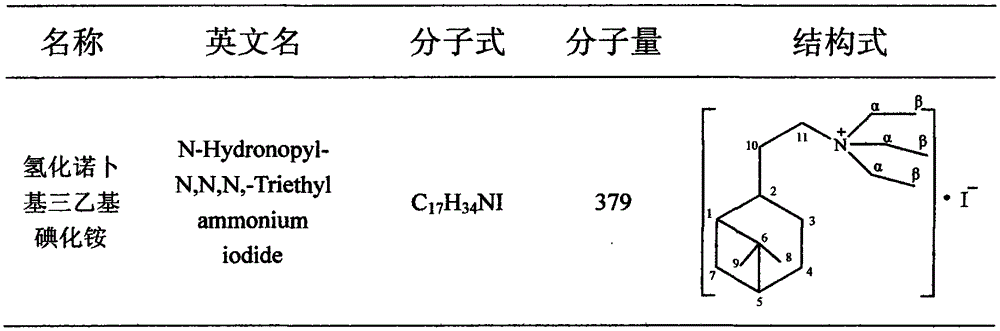
[0012] A preparation method of notyl triethylammonium iodide, comprising the following steps:
[0013] 1) In a 500ml three-necked flask equipped with a mechanical stirrer and a reflux condenser, put 100ml of acetone, 0.2mol of nobyl chloride hydrochloride and 35g of sodium iodide, stir, and heat to reflux. After 8 hours, samples were taken for GC analysis. After the conversion of alcohol is complete, cool to room temperature and filter to remove salt. After treatment, a yellowish hydronobyl iodide was obtained.
[0014] 2) Install a reflux condenser on the 250ml ground-mouth Erlenmeyer flask, place the Erlenmeyer flask on a magnetic stirrer, add 0.1mol hydronobyl iodide, 45ml acetonitrile and 0.11mol trie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com