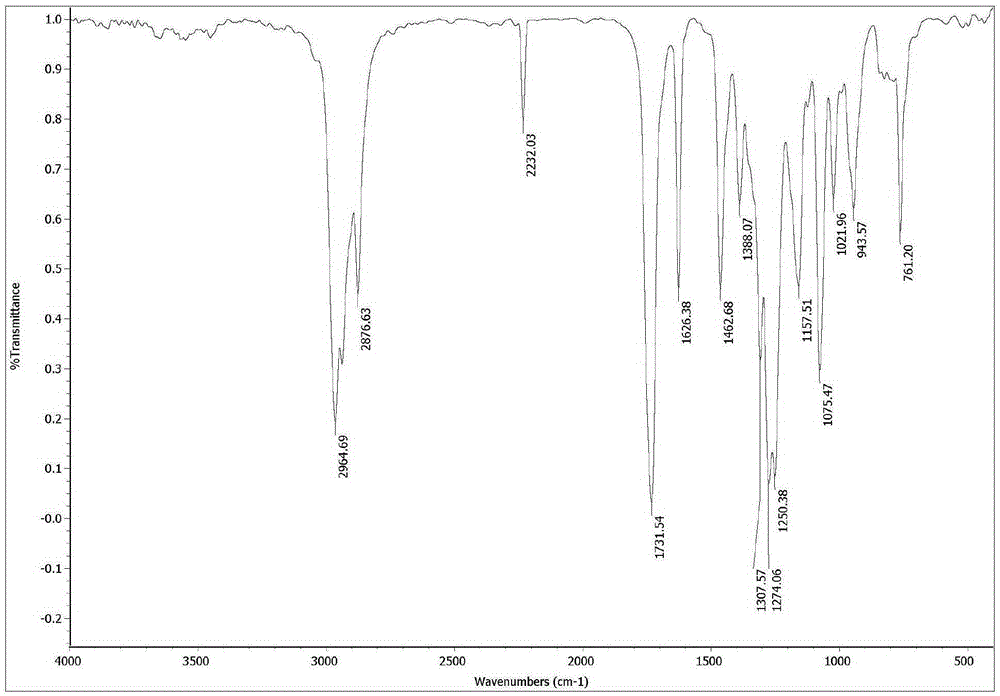
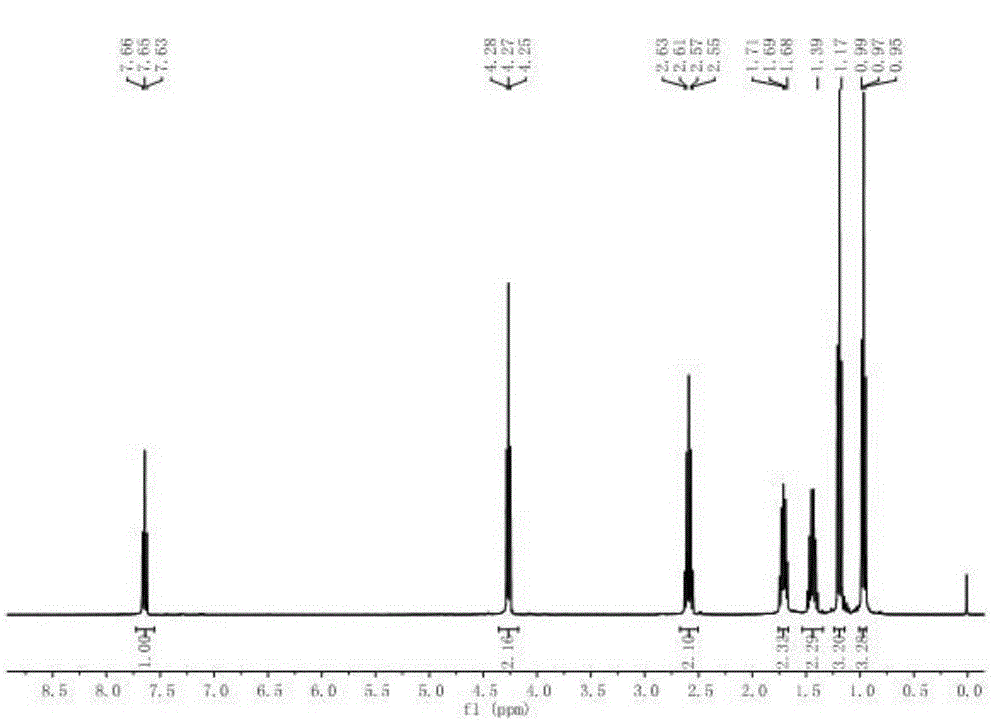
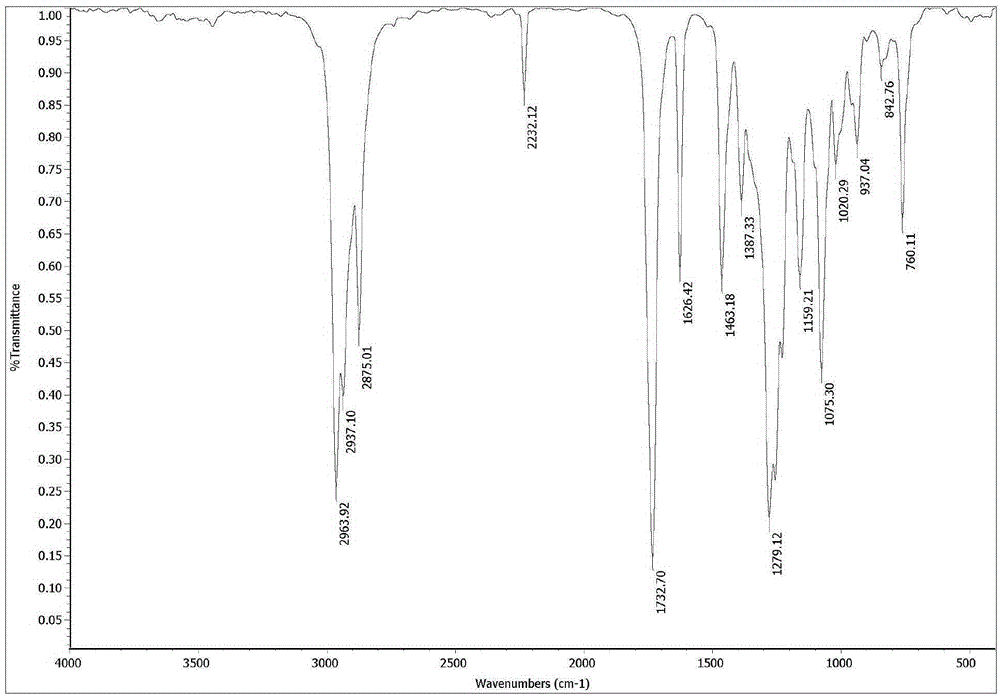
α-Butyl cyanoacrylate derivative and preparation method thereof
A technology of butyl cyanoacrylate and butyl cyanoacetate, which is applied in the field of α-butyl cyanoacrylate derivatives and its preparation, can solve the problems of different degradation speeds, unsatisfactory compatibility, cohesive force and fluidity Insufficient adjustment and other problems, to achieve the effect of short reaction time, cheap and easy-to-obtain catalyst, and low viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation method of α-cyano-α-pentenoic acid butyl ester described in the present embodiment comprises the steps:
[0045] (1) In a 250mL two-necked flask equipped with a magnetic stirrer, add butyl cyanoacetate 35.3g (250mmol), zinc acetate dihydrate 0.68g (3mmol) and propionaldehyde 29.1g (500mmol) successively, in Stir the reaction at 60°C for 5h;
[0046] (2) Recover excess propionaldehyde under reduced pressure, then add 60mL dichloromethane, transfer the reaction solution to a 250mL separatory funnel, wash three times with 100mL brine (NaCl solution) each time, separate the organic layer, and wash with anhydrous Magnesium sulfate was dried overnight, filtered, and the filtrate was removed from the solvent with a rotary evaporator;
[0047] (3) The resulting liquid was distilled under reduced pressure, and the fraction at 105-115° C. (230 Pa) was collected to obtain 38.6 g of a colorless oily liquid with a purity of 99.2% and a yield of 84.5% (calculated as ...
Embodiment 2
[0051] The preparation method of the alpha-cyano-alpha-hexenoic acid butyl ester described in the present embodiment comprises the steps:
[0052] (1) In a 250mL two-necked flask equipped with a magnetic stirrer, add 35.3g (250mmol) of butyl cyanoacetate, 0.68g (3mmol) of zinc acetate dihydrate and 36.0g (500mmol) of butyraldehyde in turn. Stir the reaction at 60°C for 5h;
[0053] (2) Recover excess propionaldehyde under reduced pressure, then add 60mL dichloromethane, transfer the reaction solution to a 250mL separatory funnel, wash three times with 100mL brine (NaCl solution) each time, separate the organic layer, and wash with anhydrous Magnesium sulfate was dried overnight, filtered, and the filtrate was removed from the solvent with a rotary evaporator;
[0054] (3) The resulting liquid was distilled under reduced pressure, and the fraction at 105-115° C. (230 Pa) was collected to obtain 38.6 g of a colorless oily liquid with a purity of 99.2% and a yield of 84.5% (calc...
Embodiment 3
[0058] The preparation method of α-cyano-α-pentenoic acid butyl ester described in the present embodiment comprises the steps:
[0059] (1) In a three-necked flask equipped with a thermometer, reflux condenser and mechanical stirring, add 70.8g (502mmol) of butyl cyanoacetate, 1.6g (7mmol) of zinc acetate dihydrate and 30.8g (630mmol) of propionaldehyde, methanol 100mL, reflux and stir at 70±5℃ for 2h;
[0060] (2) dry overnight with anhydrous magnesium sulfate, filter, and the filtrate is desolvated with a rotary evaporator;
[0061] (3) The resulting liquid was distilled under reduced pressure, and the fraction at 110-115° C. (230 Pa) was collected to obtain 83.2 g of a colorless oily liquid with a purity of 99.4% and a yield of 90.9% (calculated as butyl cyanoacetate).
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com