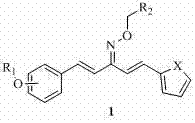
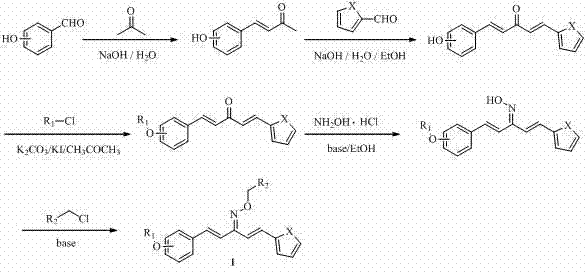
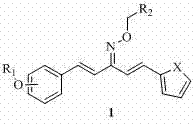
Heterocyclic 1,4-pentadiene-3-ketoxime compound as well as preparation method and application thereof
A compound and pentadiene technology, applied in the field of 1-aryl-5-heterocyclyl-1,4-pentadien-3-one oxime ether compounds, can solve problems such as uninvolved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1, 2-chloro-benzyl-1-[2-(3-fluorobenzyloxy)phenyl]-5-(2-thiophene)-1,4-pentadiene-3-ketoxime ether ( Compound A):
[0071] (1) Synthesis of 4-(2-hydroxyphenyl)-3-buten-2-one
[0072] Add 7.33 g (60 mmol) of o-hydroxybenzaldehyde (60 mmol) and 60 mL of acetone to a 500 mL three-necked flask, and stir until dissolved; slowly add about 5% sodium hydroxide solution 100 mL (132 mmol sodium hydroxide); stirring at room temperature, TLC tracking reaction, volume ratio, petroleum ether: ethyl acetate=2:1, after 10 h of reaction, dilute hydrochloric acid was added dropwise until the color of the solution did not change (pH was about 5), and there was yellow The solid precipitated out, stood overnight, filtered with suction, and dried naturally to obtain a yellow solid. Yield: 89%; Melting point: 139-141 °C.
[0073] (2) Preparation of 1-(2-hydroxyphenyl)-5-(2-thiophene)-1,4-pentadien-3-one
[0074] Add 3.57 g of 4-o-hydroxyphenyl-3-buten-2-one (22 mmol), 2.24 g of 2-...
Embodiment 2
[0081] Example two, 4-nitro-benzyl-1-[2-(benzyloxy)phenyl]-5-(2-thiophene)-1,4-pentadiene-3-ketoxime ether (compound D ):
[0082] (1) Synthesis of 4-(2-hydroxyphenyl)-3-buten-2-one
[0083] Synthesize as in Example 1 (1) method and conditions.
[0084] (2) Preparation of 1-(2-hydroxyphenyl)-5-(2-thiophene)-1,4-pentadien-3-one
[0085] Synthesize as in Example 1 (2) method and conditions.
[0086] (3) Preparation of 1-[2-(benzyloxy)phenyl]-5-(2-thiophene)-1,4-pentadien-3-one
[0087] Add 1.28 g 1-o-hydroxyphenyl-5-(2-thiophene)-1,4-pentadien-3-one (5 mmol), 1.04 g K 2 CO 3 (7.5 mmol), 0.25 g KI (1.50 mmol), 20 mL of acetone, stirred; dropwise added 5 mL of acetone containing 0.76 g benzyl chloride (6.00 mmol), heated to reflux; TLC tracking reaction, volume ratio, petroleum ether:ethyl acetate Ester = 3:1, 5 h after the reaction, cooled to room temperature; filtered, the filtrate was rotary evaporated to remove acetone, and recrystallized from ethanol to obtain yellow ne...
Embodiment 3
[0092] Example three, 4-nitro-benzyl-1-[4-(benzyloxy)phenyl]-5-(2-furan)-1,4-pentadiene-3-ketoxime ether (compound I ):
[0093] (1) Synthesis of 4-(4-hydroxyphenyl)-3-buten-2-one
[0094] Add 7.33 g (60 mmol) of 4-hydroxybenzaldehyde and 60 mL of acetone to a 500 mL three-necked flask, and stir until dissolved; slowly add about 5% sodium hydroxide solution 110 mL (150 mmol sodium hydroxide, 110 mL water); stirring at room temperature, TLC tracking reaction, volume ratio, petroleum ether: ethyl acetate = 2:1, after 16 h of reaction, dilute hydrochloric acid was added dropwise until the color of the solution did not change (pH approx. 5), a yellow solid was precipitated, left to stand overnight, filtered with suction, and dried naturally to obtain a yellow powder. Yield: 80%; Melting point: 96-98 °C.
[0095] (2) Preparation of 1-(4-hydroxyphenyl)-5-(2-furan)-1,4-pentadien-3-one
[0096] Take 3.57 g 4-(4-hydroxyphenyl)-3-buten-2-one (22 mmol), 1.92 g 2-furaldehyde (20 mmol)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com