5-methyl-1,2,3-thiadiazole-containing 1,3,4-thiadiazole derivatives and their preparation method and use
A thiadiazole derivative, thiadiazole technology, applied in the field containing 1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
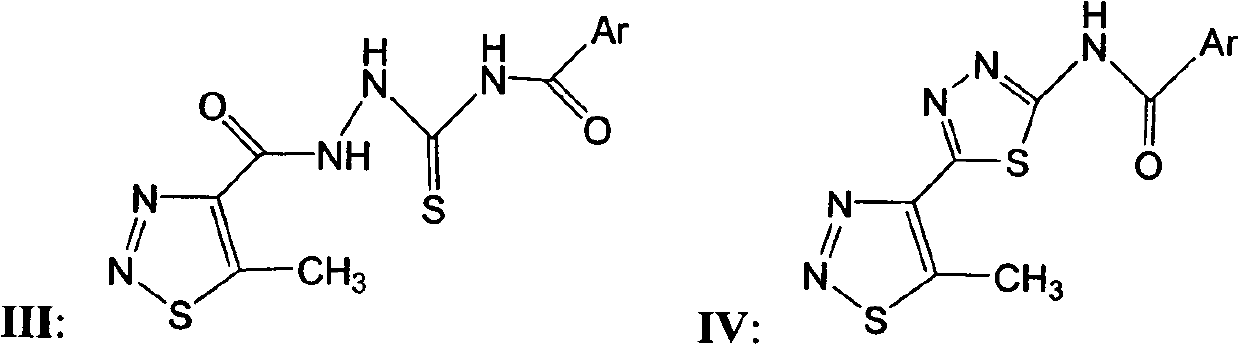
[0027] Preparation of intermediate N-(2-(5-methyl-1,2,3-thiadiazole-4-carboxhydrazino)thioacyl) substituted benzamide III:
[0028] Add 3.33 millimoles of 5-methyl-1,2,3-thiadiazole-4-carboxamide I, 3.33 millimoles of substituted thioisocyanate II and 20 milliliters of DMF into a 50 milliliter three-necked round bottom flask, Room temperature; stir for 6 hours. A white solid appears. The filtered solid ethanol is recrystallized with a yield of 60%-90%. The melting point and 1 H NMR, the chemical structure and physical and chemical parameters of compound III are shown in Table 1.
Embodiment 2
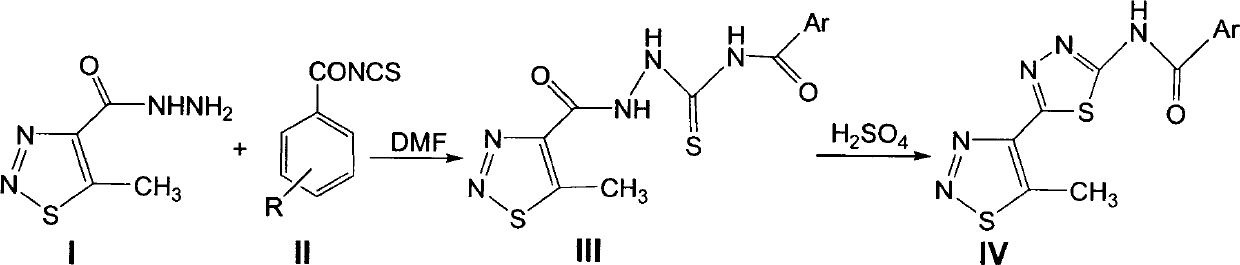
[0030] Preparation of 5-methyl-1,2,3-thiadiazole and -1,3-4-thiadiazole IV:
[0031] Add 3.0 mmoles of N-(2-(5-methyl-1,2,3-thiadiazole-4-carboxhydrazino)thioacyl) substituted benzamide III into a 50 ml in-situ flask. 20 methanol, 0.1 ml concentrated sulfuric acid, stir and reflux for 6 hours. A white solid appears. The filtered solid ethanol is recrystallized with a yield of 60%-90%. The melting point and 1 H NMR, the chemical structure and physical and chemical parameters of compound IV are shown in Table 1.
Embodiment 3
[0033] The antibacterial or bactericidal activity of the 5-methyl-1,2,3-thiadiazole-linked-1,3-4-thiadiazole derivative IV and its intermediate III of the present invention:
[0034] The names and codes of common plant pathogenic fungi tested in the present invention include AS: Alternaria solani; BC: Botrytis cinerea; CA: Cercospora arachidicola; GZ: Wheat red Gibberella zeae; PI: Phytophthora infestans (Mont.) de Bary; PP: Physalospora piricola; PS: Pellicularia sasakii; RC: Gramineae Rhizoctonia cerealis (Rhizoctonia cerealis); SS: Sclerotinia sclerotiorum (Sclerotinia sclerotiorum), these strains are very representative and can represent most of the pathogen species that occur in the field in agricultural production. The measurement results of the bacterial growth rate method are shown in Table 2. Table 2 shows that at 50 μg / ml, all the compounds synthesized in the present invention have different degrees of bactericidal activity, and the bactericidal activity of TN-TDZ9 and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com