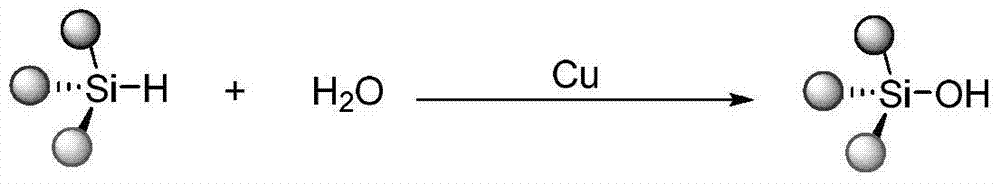
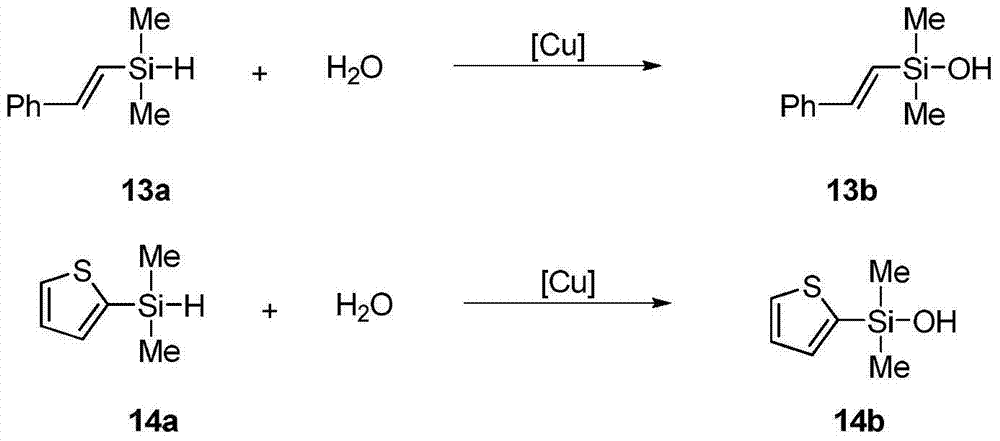
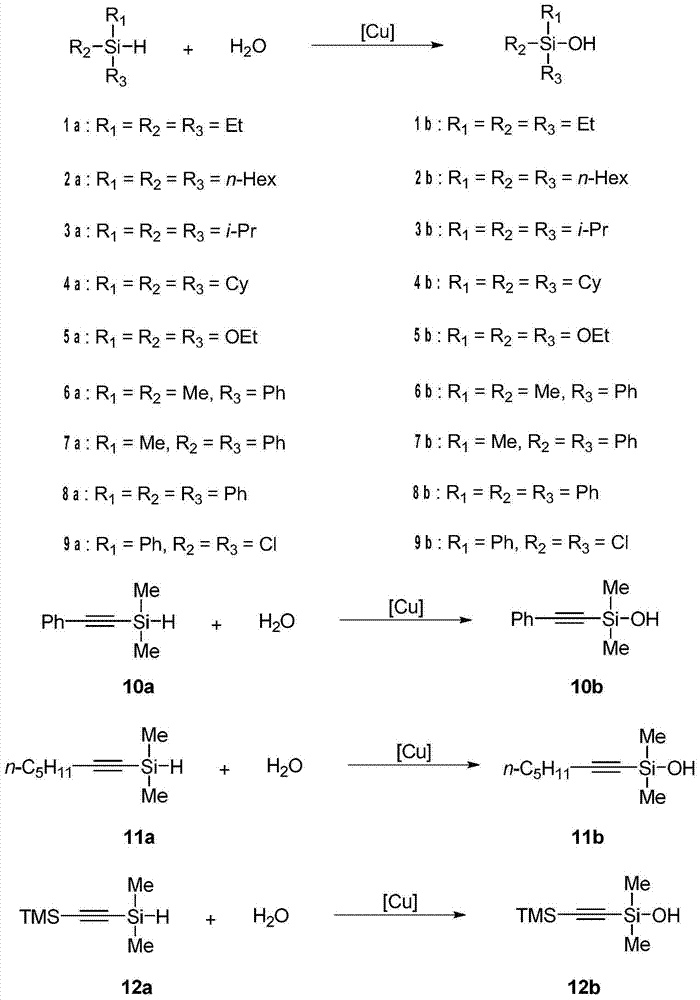Method for catalytically synthesizing silanol
A technology of silanol and catalyst, which is applied in the field of catalyzing synthesis of silanol compounds with water as an oxidant copper salt, can solve the problems of unsuitability for large-scale production, low selectivity, narrow application range, etc., and achieves easy separation and simple product separation method. , the effect of less emission of "three wastes"
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Mix triethylsilane 1a (2mmol) with water (2mmol), use copper sulfate as a catalyst, the catalyst dosage is 1mol% of the moles of triethylsilane, react in the air without solvent, stir and heat to 50°C, and the reaction time is 3 hours. After the reaction, 20 mL of water was added, extracted with ethyl acetate, dried, and the solvent was distilled off under reduced pressure to obtain colorless liquid triethylsilanol 1b. The conversion of silane was 100%, and the yield of triethylsilanol 1b was 93%.
Embodiment 2
[0028] Mix trihexylsilane 2a (2mmol) with water (2mmol), add copper sulfate as a catalyst, the amount of catalyst is 1mol% of the moles of trihexylsilane, react in the air without solvent, stir and heat to 50°C, and the reaction time is 3 hours . After the reaction, 10 mL of water was added, extracted with ethyl acetate, dried, and the solvent was distilled off under reduced pressure to obtain trihexylsilanol 2b as a colorless liquid. The conversion of silane was 100%, and the yield of trihexylsilanol 2b was 91%.
Embodiment 3
[0030] Mix triisopropylsilane 3a (2mmol) with water (2mmol), add copper oxide as a catalyst, the catalyst dosage is 10mol% of the moles of triisopropylsilane, react without solvent under air, stir and heat to 50°C, react The time is 3 hours. After the reaction, 15 mL of water was added, extracted with ethyl acetate, dried, and the solvent was distilled off under reduced pressure to obtain colorless liquid triisopropylsilanol 3b. The conversion of triisopropylsilane was 100%, and the yield of triisopropylsilanol 3b was 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com