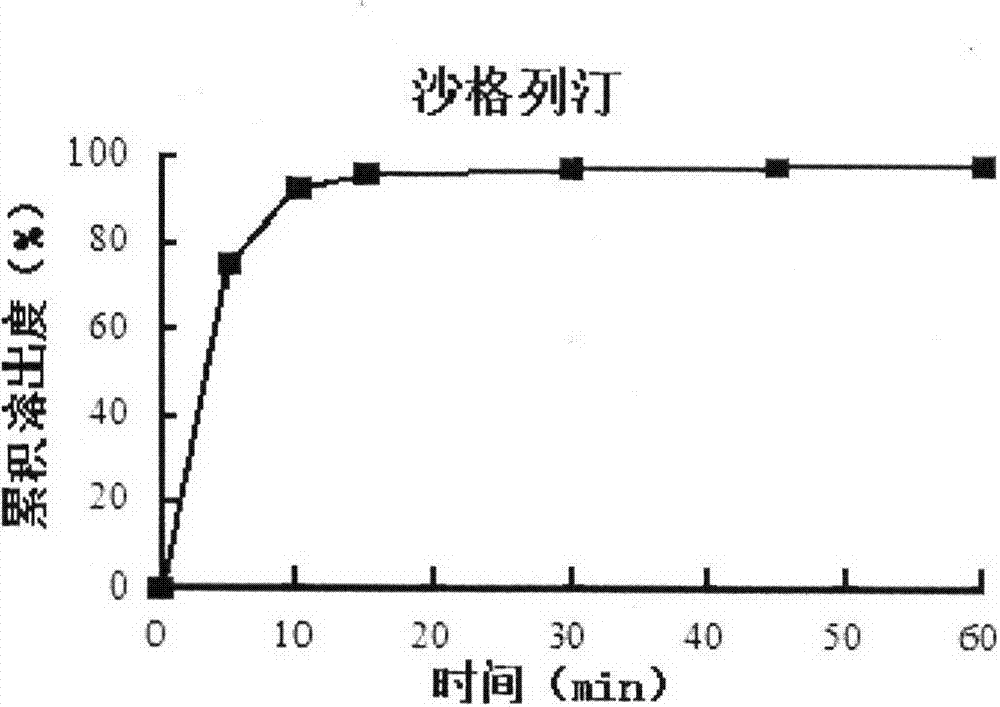
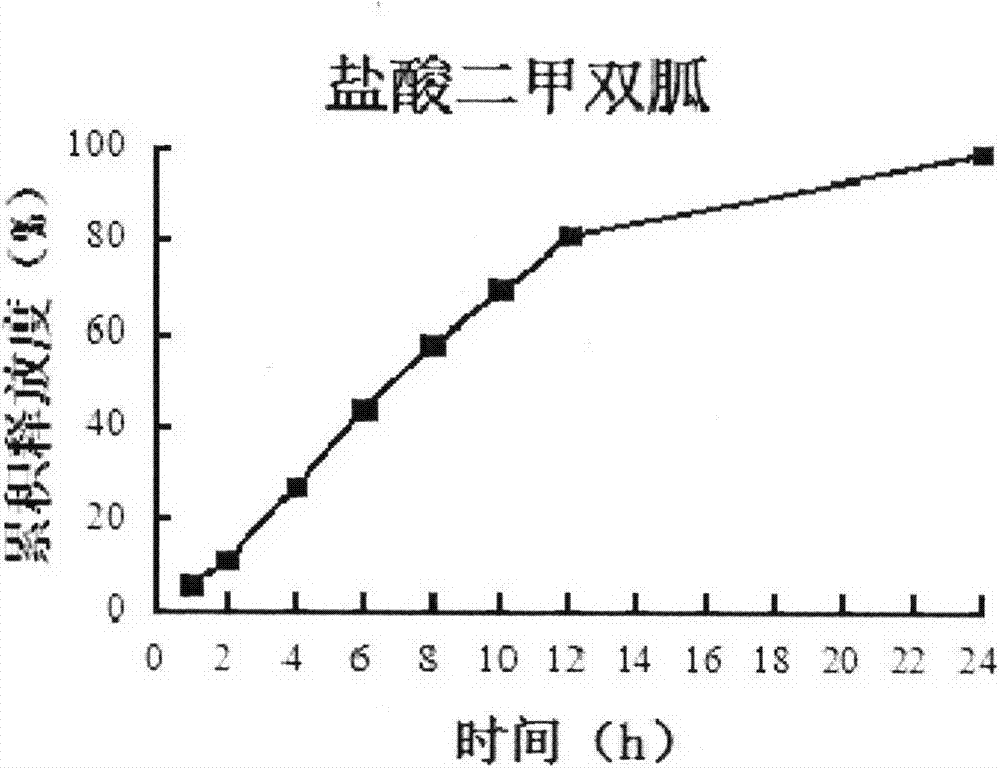
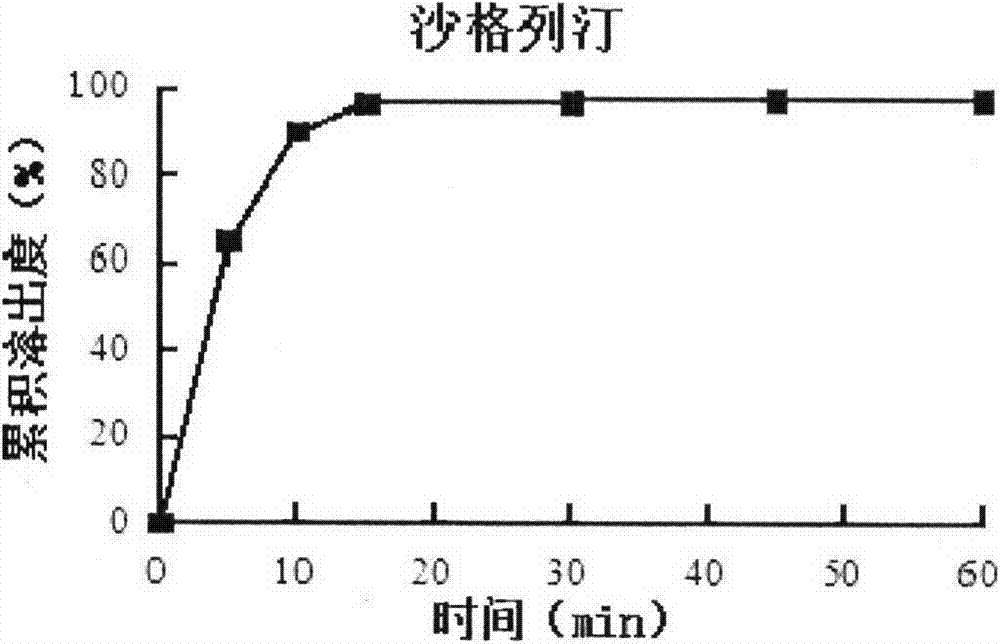
Compound preparation including DPP-4 inhibitor and metformin hydrochloride and preparation method thereof
A technology of metformin hydrochloride and DPP-4, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., and can solve the problems that blood drug concentration is not as stable as controlled-release tablets, poor patient compliance, and short half-life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 (metformin hydrochloride / saxagliptin, 500 / 5mg)
[0047] Metformin Hydrochloride Osmotic Pump Controlled Release Tablet Core Prescription (1000 Tablets)
[0048]
[0049] Prescription of controlled release film coating solution (1000 tablets)
[0050]
[0051] Prescription of saxagliptin immediate release layer coating solution (1000 tablets)
[0052]
[0053] Preparation Process:
[0054] ①Preparation of Metformin Hydrochloride Osmotic Pump Controlled-release Tablet Core: Weigh the prescribed amount of Metformin Hydrochloride and HPMC E5, add equal amounts and mix evenly; add appropriate amount of distilled water to moisten and prepare soft material and granulate; add other remaining excipients after drying and granulating , mix evenly; tablet, hardness 10-12kg.
[0055] ② Coating of controlled-release film: Weigh the prescribed amount of cellulose acetate and glycerol triacetate, dissolve them in acetone, dissolve the prescribed amount of PEG40...
Embodiment 2
[0061] Embodiment 2 (metformin hydrochloride / saxagliptin, 500 / 5mg)
[0062] Metformin Hydrochloride Osmotic Pump Controlled Release Tablet Core Prescription (1000 Tablets)
[0063]
[0064] Prescription of controlled release film coating solution (1000 tablets)
[0065]
[0066] Prescription of saxagliptin immediate release layer coating solution (1000 tablets)
[0067]
[0068] Preparation Process:
[0069] ①Preparation of Metformin Hydrochloride Osmotic Pump Controlled-release Tablet Core: Weigh the prescribed amount of Metformin Hydrochloride and HPMC E5, add equal amounts and mix evenly; add appropriate amount of distilled water to moisten and prepare soft material and granulate; add other remaining excipients after drying and granulating , mix evenly; tablet, hardness 10-12kg.
[0070] ② Coating of controlled-release film: Weigh the prescribed amount of cellulose acetate and glycerol triacetate, dissolve them in acetone, dissolve the prescribed amount of PEG40...
Embodiment 3
[0074] Embodiment 3 (metformin hydrochloride / saxagliptin, 500 / 5mg)
[0075] Metformin Hydrochloride Osmotic Pump Controlled Release Tablet Core Prescription (1000 Tablets)
[0076]
[0077] Prescription of controlled release film coating solution (1000 tablets)
[0078]
[0079] Prescription of saxagliptin immediate release layer coating solution (1000 tablets)
[0080]
[0081]
[0082] Preparation Process:
[0083] ①Preparation of Metformin Hydrochloride Osmotic Pump Controlled-release Tablet Core: Weigh the prescribed amount of Metformin Hydrochloride and HPMC E5, add equal amounts and mix evenly; add appropriate amount of distilled water to moisten and prepare soft material and granulate; add other remaining excipients after drying and granulating , mix evenly; tablet, hardness 10-12kg.
[0084] ② Coating of controlled-release film: Weigh the prescribed amount of cellulose acetate and glycerol triacetate, dissolve them in acetone, dissolve the prescribed amo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com