Peptide vaccine used for animal and its preparation
一种合成肽疫苗、疫苗的技术,应用在口蹄疫合成肽疫苗的多肽领域,能够解决不能有效地保护动物、影响新型疫苗使用、效果差等问题,达到良好应用前景、易于大规模合成、增强免疫效果的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Solid-phase synthesis of foot-and-mouth disease synthetic peptide antigen
[0034] The polypeptide antigen of the present invention can be prepared by using an automatic polypeptide synthesizer and Merrifield solid-phase synthesis method, in which 9-fluorenylmethyloxycarbonyl (Fmoc) modified amino acids are used, and the solid-phase carrier is Rink Amide MBHA resin. The production process usually includes solid-phase synthesis of peptide antigens, peptide lysis, antigen purification and sterilization preservation.
[0035] 1.1 Solid-phase synthesis of peptide antigens
[0036] 1.1.1 Preparation of synthetic raw materials
[0037] The sequences of the synthetic polypeptide antigens are shown in SEQ ID NO. 1, SEQ ID NO. 2, SEQ ID NO. 3, SEQ ID NO. 4, SEQ ID NO. 5 and SEQ ID NO. 6, respectively.
[0038] According to the sequence of the antigen and the synthesis scale, a suitable Fmoc modified amino acid is prepared for 1 mmol and added to the corresponding amino acid via...
Embodiment 2
[0064] Example 2. Preparation of synthetic peptide vaccine
[0065] 2.1 Preparation of antigen aqueous phase
[0066] First, weigh the three polypeptide antigens synthesized according to the above-mentioned Example 1; then, dilute the synthetic peptide antigen concentration to 50μg / ml with sterile water for injection; filter the antigen solution through a filter with a pore size of 0.2μm to sterilize .
[0067] 2.2 Preparation of oil phase adjuvant
[0068] Sterilize the oil phase adjuvant at 121°C for 30 minutes, and set aside.
[0069] 2.3 Emulsification of synthetic peptide vaccine
[0070] Clean the IKA emulsification equipment 3 times with 2000ml of sterilized distilled water, and then add the oil phase to the emulsification tank at a volume ratio of 1:1 between the oil phase adjuvant and the antigen aqueous phase at 20-28°C, and start the motor After stirring at 90~150r / m slowly for 2 minutes, add the water phase antigen slowly at the same time. After adding, stir for 30 minutes,...
Embodiment 3
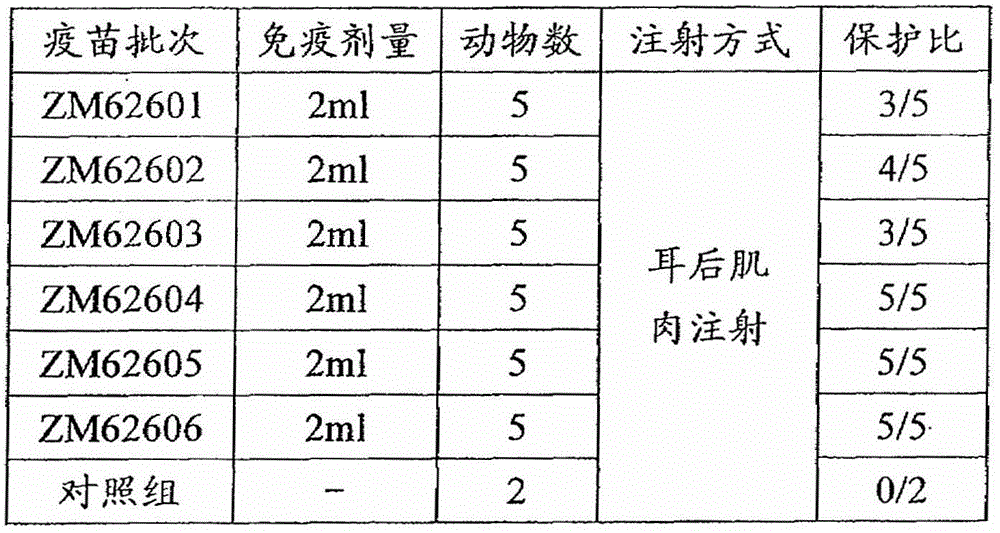
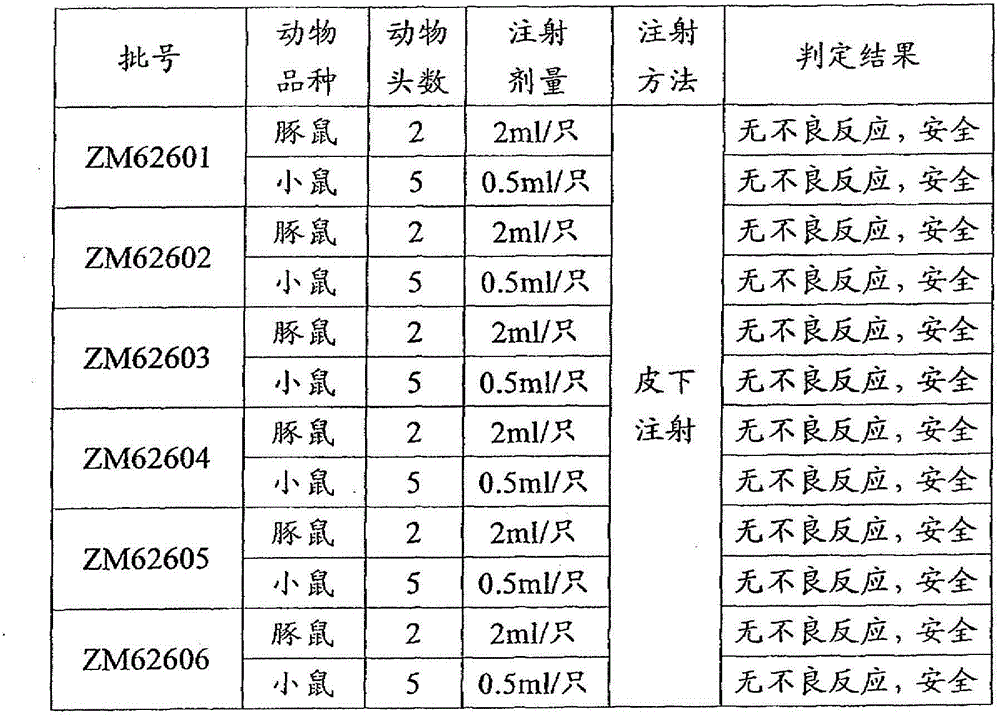
[0071] Example 3 Efficacy test of synthetic peptide vaccine
[0072] 1. Materials and methods
[0073] 1.1 Synthetic peptide vaccine
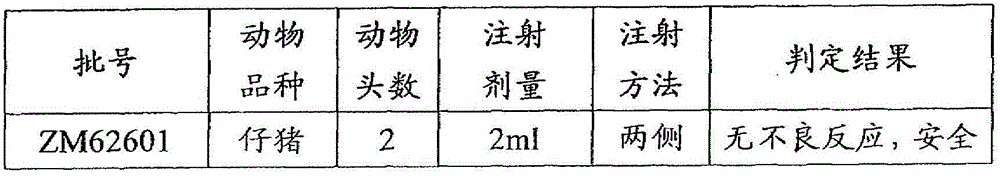
[0074] According to the above-mentioned examples, the polypeptide antigens with SEQ ID NO. 1, SEQ ID NO. 2, SEQ ID NO. 3, SEQ ID NO. 4, SEQ ID NO. 5 and SEQ ID NO. 6 were synthesized, and then respectively formulated and The corresponding batch numbers are: ZM62601, ZM626A02, ZM626A03, ZM626A04, ZM626A05, ZM626A06 foot-and-mouth disease synthetic peptide vaccine.
[0075] 1.2 Experimental animals
[0076] Select 32 healthy pigs of the same breed, 4 months old, about 40Kg weight, and negative for foot-and-mouth disease neutralizing antibodies.
[0077] 1.3 Virus species OR / 80MF 8
[0078] OR / 80MF 6 After passing through suckling mice for 2 generations, the virus was collected. After the suckling mice pass the poison test, they should be frozen and stored at -20°C for later use.
[0079] 1.4 Test method
[0080] For each group of vaccines, 5 healthy and susc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com