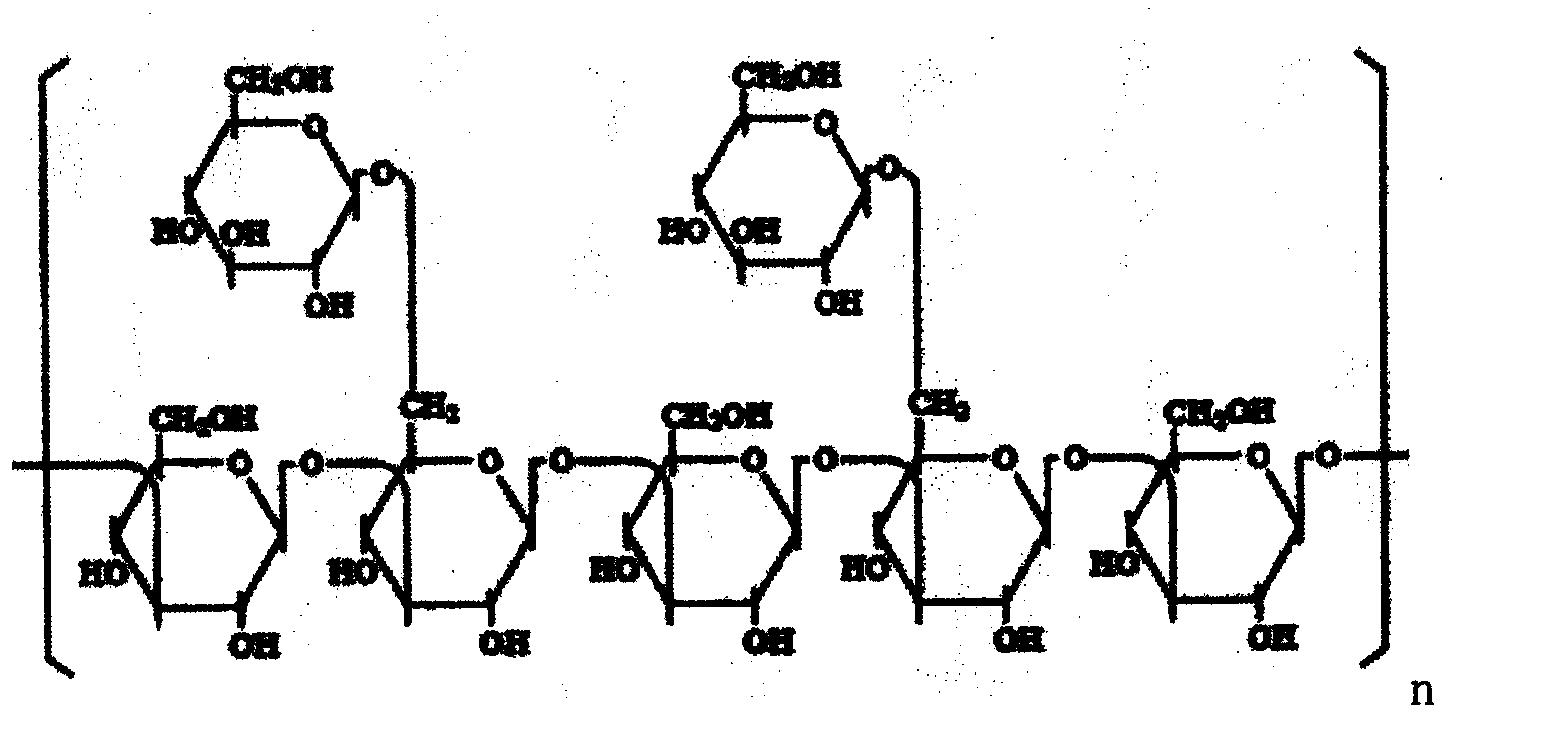
Method for dissolving lentinan solid powder
A technology of lentinan and solid powder, which is applied in the field of preparation of biological reaction regulators, can solve the problems of unavoidable patient toxicity and side effects, reduce the curative effect of lentinan, and achieve the effects of avoiding adverse reactions, improving safety, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 0.1g of lentinan solid powder into the prepared 1000ml of water, and shear with a shearing machine at a speed of 500rpm and a shearing time of 0min to obtain a uniformly dispersed liquid. Pour the uniformly dispersed liquid after high-speed shearing into the feed port of the homogenizer. Firstly, use the second-stage homogenizing valve to adjust the pressure to 30 bar and homogenize for 0 times. Then use the first-stage homogenizing valve to adjust the pressure to 300bar, homogenized twice to obtain a colorless and clear lentinan solution with a total concentration of 0.1mg / ml.
Embodiment 2
[0019] Add 0.1g of lentinan solid powder into the prepared 1000ml of water, and shear with a shearing machine at a speed of 15,000rpm and a shearing time of 15min to obtain a uniformly dispersed liquid. Pour the uniformly dispersed liquid after high-speed shearing into the feed port of the homogenizer. Firstly, use the second-stage homogenizing valve to adjust the pressure to 150 bar, homogenize twice, and then use the first-stage homogenizing valve to adjust the pressure to 1350bar, homogenized 5 times to obtain a colorless and clear lentinan solution with a total concentration of 0.1mg / ml.
Embodiment 3
[0021] Add 0.1g of lentinan solid powder into 1000ml of water that has been prepared, and shear with a shearing machine at a speed of 30,000rpm and a shearing time of 30min to obtain a uniformly dispersed liquid. Pour the uniformly dispersed liquid after high-speed shearing into the feed port of the homogenizer, first use the second-stage homogenizing valve, adjust the pressure to 300bar, homogenize for 3 times, and then use the first- and second-stage homogenizing valves to adjust the pressure 2000bar, homogenized 7 times to obtain a colorless and clear lentinan solution with a total concentration of 0.1mg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com