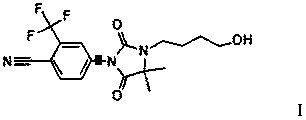
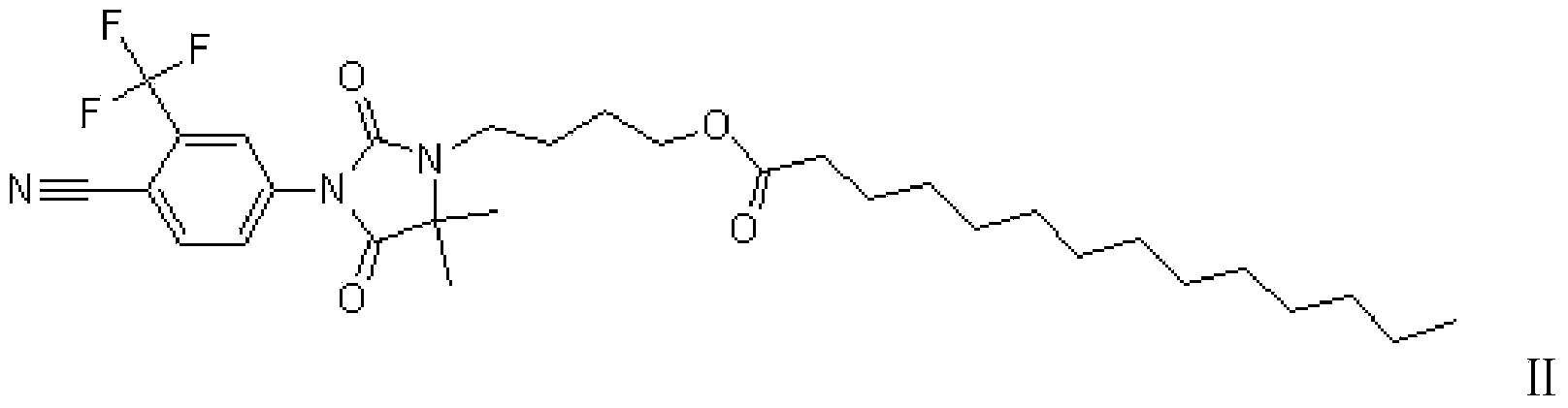
Compound with antiandrogen activity as well as preparation method and application of compound
An anti-androgen and compound technology, applied in the field of biomedicine, can solve problems such as limiting general application, and achieve the effect of good curative effect and good bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 10g of compound IV (33.6mmol) and 10g of compound V (51mmol) were added to 50ml of DMF under stirring between 20°C and 25°C, and stirred until dissolved, then 2.5g of sodium metal (0.1 mol). The reaction solution was stirred and reacted at room temperature for 24 hours, and then 2.5 ml of saturated ammonium chloride solution was added to the reaction solution to quench the sodium metal. The resulting reaction solution was poured into 250ml of ice water, stirred to precipitate precipitates, suction filtered, and the filter cake was vacuum-dried and then recrystallized in 200ml of ethanol to obtain 3.73g of product compound III with a yield of 27%.
Embodiment 2
[0023] Between 0°C and 5°C, 2.5g of sodium hydrogen (0.1mol) was added in batches to 20ml of DMF under stirring, and the solution formed by 10g of compound IV (33.6mmol) and 30ml of DMF was added dropwise to sodium hydrogen and In the suspension formed by DMF, 10 g of compound V (51 mmol) was added after the dropwise addition. The reaction solution was stirred at room temperature for 24 hours, and then 2.5 ml of saturated ammonium chloride solution was added to the reaction solution to quench the sodium hydrogen. The resulting reaction solution was poured into 250ml of ice water, stirred to precipitate the precipitate, filtered with suction, and the filter cake was vacuum-dried and then recrystallized in 200ml of ethanol to obtain 4.05g of compound III with a yield of 29.3%.
Embodiment 3
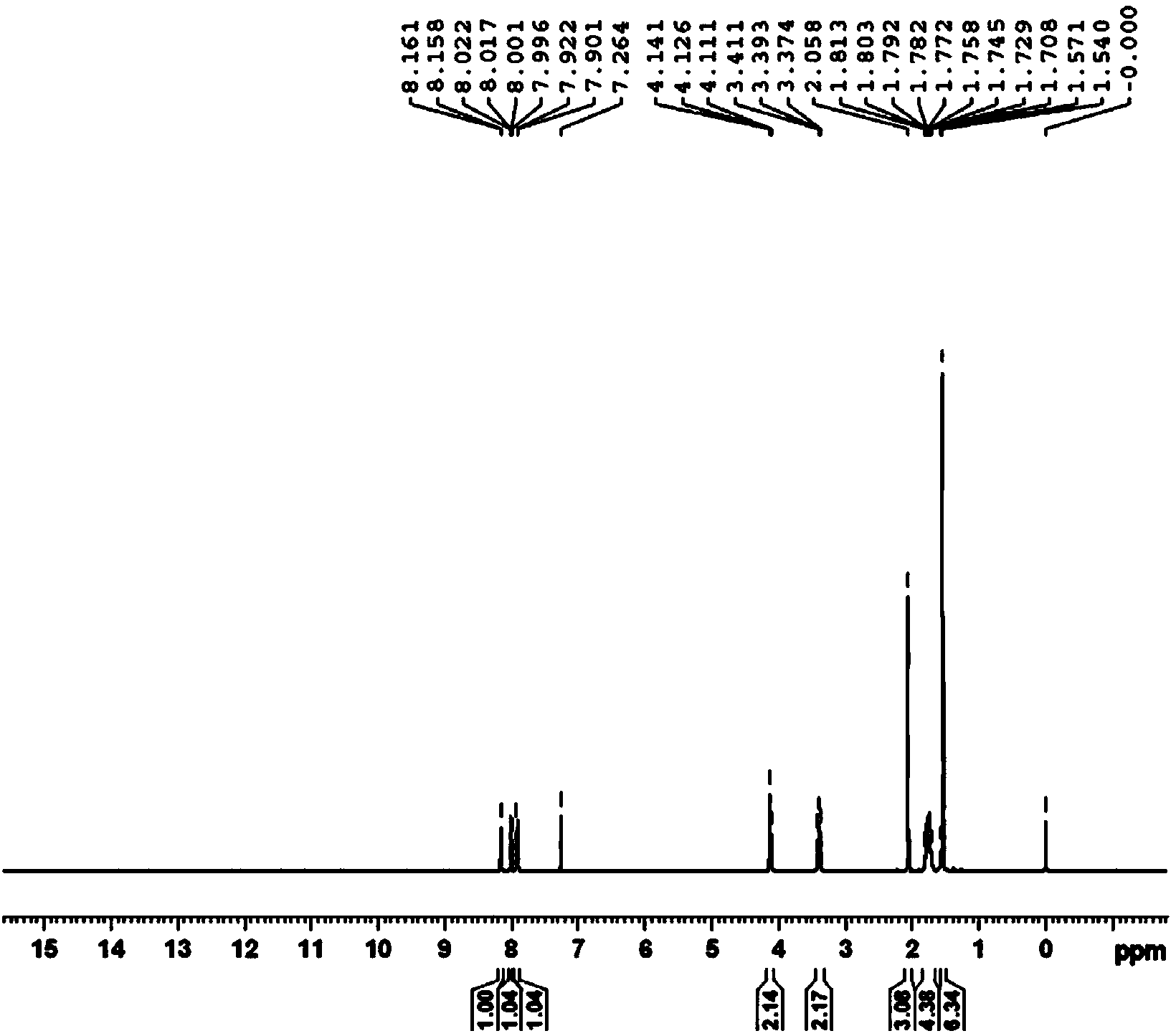
[0025] 10g of compound IV (33.6mmol) and 10g of compound V (51mmol) were added between 20°C and 25°C in 50ml of DMF under stirring, and after stirring until dissolved, 10g of potassium carbonate (72.4mmol) was added to the reaction solution ). The reaction solution was stirred at room temperature for 72 hours. The resulting reaction solution was poured into 250ml of ice water, stirred to precipitate precipitates, filtered with suction, and the filter cake was vacuum-dried and then recrystallized in 500ml of ethanol to obtain 11.29g of compound III with a yield of 81.6%. Melting point: 99℃~101℃. NMR confirmed the structure. H NMR (CDCl3, 400MHz) δ1.54(s, 6H, CH3), 1.77(m, 4H, CH2), 2.06(s, 3H, CH3), 3.39(m, 2H, CH2), 4.12(m, 4H , CH2), 7.91 (d, 1H, CH), 8.01 (dd, 1H, CH), 8.16 (d, 1H, CH). The solubility of compound III in ethanol reaches 2.5g / 100g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com