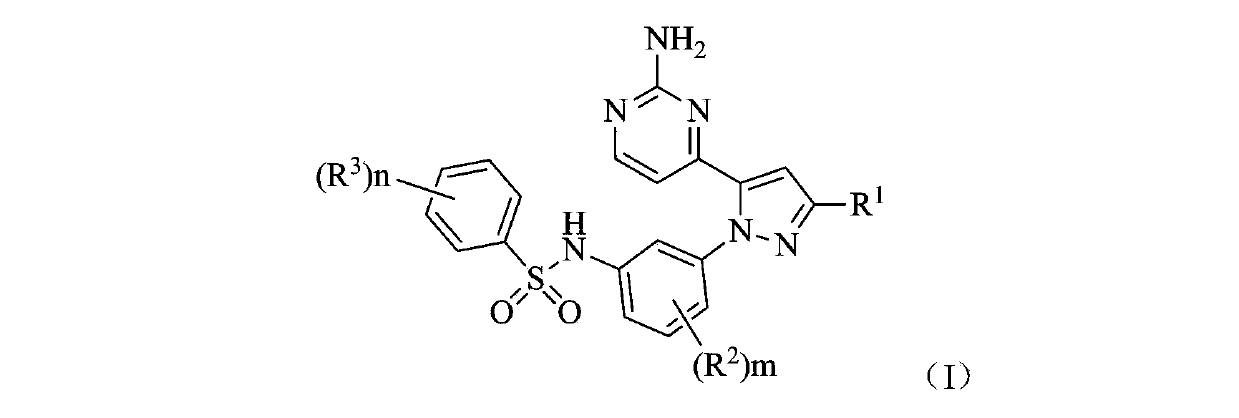
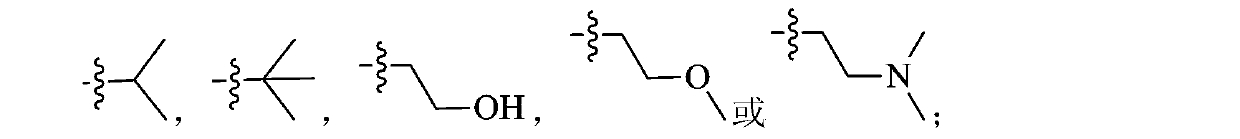
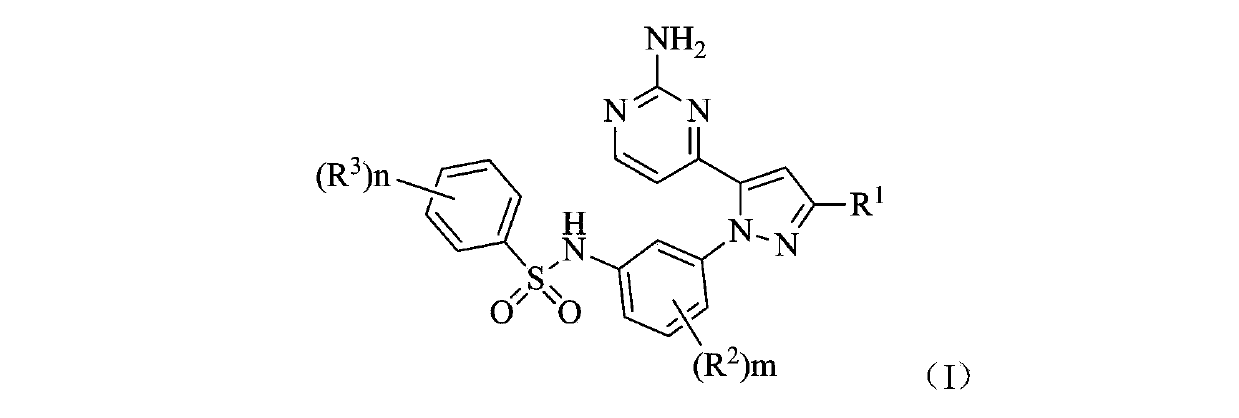
Benzene sulfonamide pyrazole kinase inhibitor
A carbamoyl and alkyl technology, applied in the field of benzenesulfonamide pyrazole kinase inhibitors, can solve problems such as poor selectivity and insufficient activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] Step 1 Preparation of TMa
[0068] To an appropriate amount of 1,4-dioxane, add an appropriate amount of SM1, SM2, potassium acetate and [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium dichloromethane complex in sequence, and react Under nitrogen protection, stir at 90°C for 16 hours, cool and concentrate under reduced pressure. The obtained solid is dissolved in ethyl acetate, washed with water, and the organic phase is dried with anhydrous sodium sulfate. After concentrated under reduced pressure, the product is separated and purified by silica gel column chromatography. TMa.
[0069] Step 2 Preparation of TMb
[0070] Dissolve TMa in a mixed solution of tetrahydrofuran and water, and add an appropriate amount of NaIO 4 (sodium periodate), stir at room temperature until the system becomes homogeneous, stir overnight at room temperature, remove the solvent under reduced pressure, dissolve the crude product in ethyl acetate, wash with water and saturated aqu...
experiment example
[0099] Experimental example The in vitro enzymatic activity experiment of the compound of the present invention
[0100] Test product: Compound 1 prepared in Example 1.
[0101] experimental method:
[0102] The meanings of the English and English abbreviations in the following tests are as follows:
[0103] HEPES: Hydroxyethylpiperazineethanesulfonic acid;
[0104] Brij-35: lauryl polyethylene glycol ether;
[0105] EDTA: ethylenediaminetetraacetic acid;
[0106] Fluorescein-MAP2K1: Fluorescein-labeled MAP2K1;
[0107] ATP: adenosine triphosphate;
[0108] DMSO: dimethyl sulfoxide;
[0109] MgCl 2 : Magnesium chloride.
[0110] 1. Preparation of test reagents
[0111] ① 1X kinase buffer (50 mM HEPES, pH 7.5, 10 mM MgCl 2 , 1mM EGTA, 0.01% Brij-35);
[0112] ② 2x kinase solution (add corresponding kinase to 1x kinase buffer to prepare 2x kinase solution, the final concentration is b-RAF 3.5 nM, b-RAF V599E 0.35 nM);
[0113] ③ 4-fold substrate solution (add Fluore...
Embodiment 1
[0129] Example 1 N-(3-(5-(2-aminopyrimidin-4-yl)-3-tert-butyl-1H-pyrazol-1-yl)-2-fluorophenyl)-2,6-di Preparation of fluorobenzenesulfonamide (compound 1)
[0130]
[0131] (1) Preparation of 2-(2-fluoro-3-nitrophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
[0132]
[0133] To 1,4-dioxane (20 mL), add 1-bromo-2-fluoro-3-nitrobenzene (2.2 g, 0.01 mol), bispinacol borate (3.81 g, 0.015 mol ), potassium acetate (2.94 g, 0.03 mol) and [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium dichloromethane complex (100 mg), the reaction was carried out under nitrogen protection at Stir at 90°C for 16 hours, cool and concentrate under reduced pressure, dissolve the obtained solid in ethyl acetate, wash with water, dry the organic phase with anhydrous sodium sulfate, concentrate under reduced pressure, and use silica gel column chromatography (petroleum ether: ethyl acetate=2 : 1) Separate and purify the product (1.24 g, yield 46%).
[0134] (2) Preparation of 2-fluoro-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com