Chimeric chemokines receptors capable of making T cells tend to tumor locations
A tumor and factor technology, applied in the fields of molecular biology and immunology, can solve problems such as complex structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
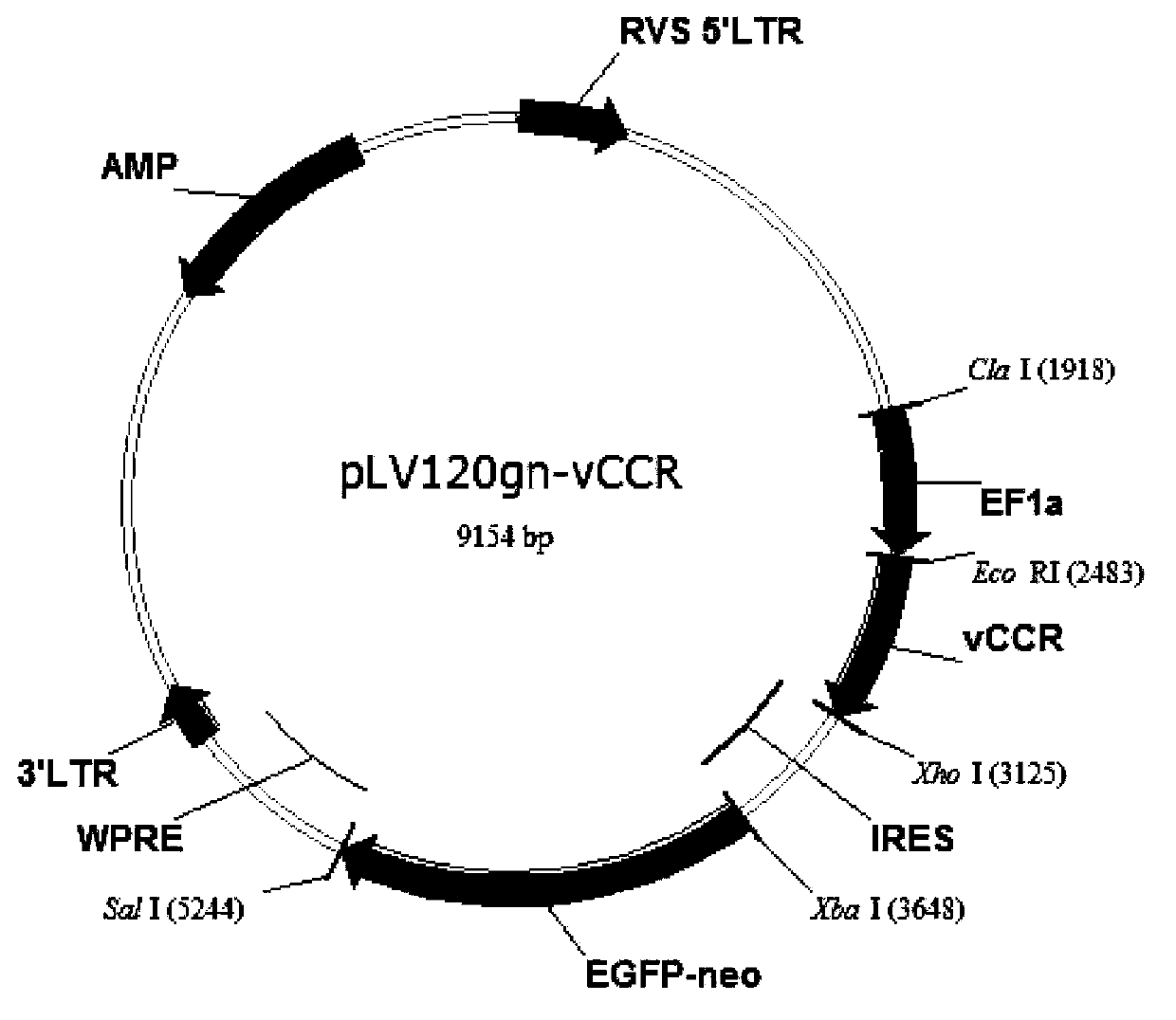
[0064] Example 1: Design, synthesis and construction of expression vector of vCCR expression cassette
[0065] According to the amino acid sequence and coding sequence of each component of vCCR, splicing into the entire fused amino acid sequence and coding DNA expression frame, wherein the peptides constituting vCCR include:
[0066] The amino acid residue sequence of the signal peptide is:
[0067] MEFWLSWVFLVAILKGVQC (SEQ ID NO: 1).
[0068] The signal peptide coding sequence is:
[0069] GAGTTTTGGCTGAGCTGGGTTTTCCTTGTTGCTATTTTAAAAGGTGTCCAGTGT (SEQ ID NO: 2).
[0070] The amino acid residue sequence of Linker1 is:
[0071] GGGGGGGGG (SEQ ID NO: 3).
[0072] The coding sequence of Linker1 is:
[0073] GGTGGAGGTGGAGGTGGAGGTGGAGGT (SEQ ID NO: 4).
[0074] The amino acid residue sequence of Linker2 is:
[0075] GGGGSGGGGS (SEQ ID NO: 5).
[0076] The coding sequence of Linker2 is:
[0077] GGTGGCGGAGGCTCCGGAGGTGGAGGCTCT (SEQ ID NO: 6).
[0078] The amino acid residue ...
Embodiment 2
[0096] Example 2: Genetic modification of T cell lines
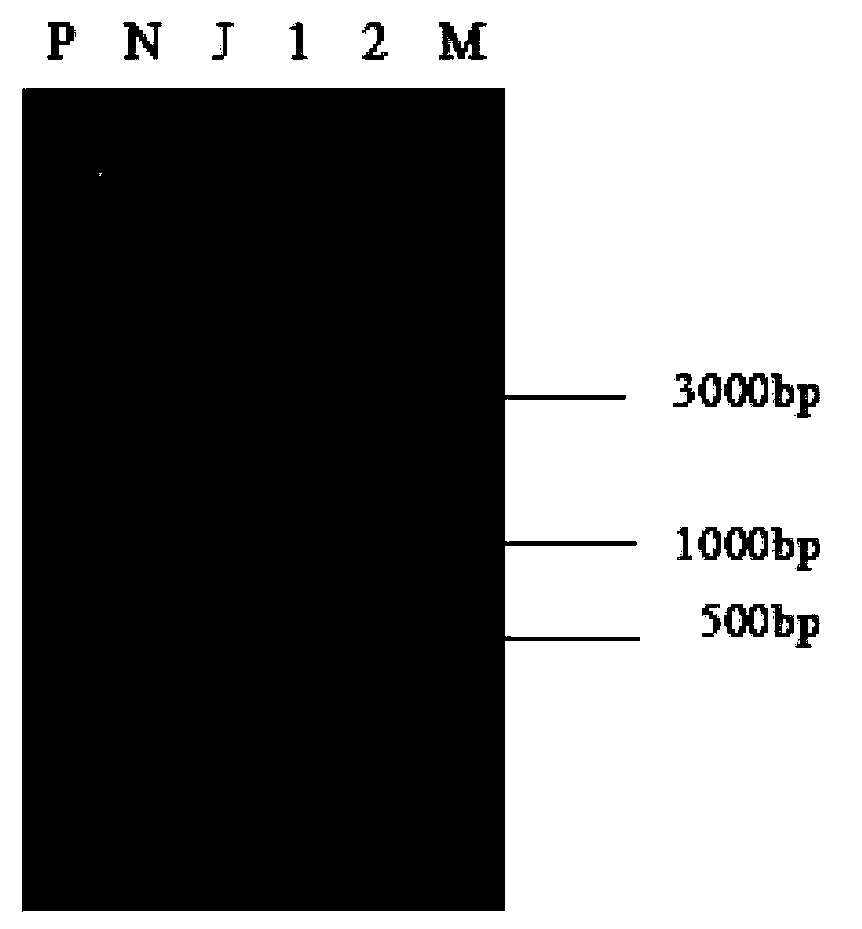
[0097] The high-quality plasmids of each recombinant expression vector constructed and purified in Example 1 were transfected into Jurkat (T lymphocyte cell line, purchased from Hong Kong University) by calcium phosphate transfection method. After 3 days, the transfected Jurkat cells were transferred to RPMI1640 medium with neomycin, and the cells were cloned by limiting dilution method. After 36 days of selection, Jurkat cell lines (Clone1, Clone2) with neomycin resistance and genetic modification of vCCR were established (see image 3 ).
[0098] Primers for synthetic PCR identification of the vCCR expression cassette:
[0099] Upstream primer 5'-TGGCTCTCCTCAAGCGTATT-3' (SEQ ID NO: 15),
[0100] Downstream primer 5'-TGCTCAGGTAGTGGTTGTCG-3' (SEQ ID NO: 16).
[0101] The predicted product size is 744bp.
[0102] Follow the instructions of the Biomed Cell Genomic DNA Rapid Extraction Kit (Cat. No.: DN0701) to extra...
Embodiment 3
[0103] Example 3: Flow cytometry detection of Jurkat cell lines genetically modified by vCCR (Clone1, Clone2) expression rate
[0104] Jurkat cells were treated without adding antibodies to adjust the voltage according to the method, Jurkat plus flow antibody PE was used as a control group, the sample group was the screened Clone1 plus flow antibody PE, and the screened Clone2 plus flow antibody PE; cell count, adjusted Concentration, take 10 6 -10 7 For each cell, wash once with 1×PBS; wash once with 1×PBS, centrifuge to remove supernatant, resuspend in 100 μl PBS; add 10 μg mouse IgG and incubate for 15 minutes; add 10 μl PE-conjugated anti-FLT1 reagent (purchased from R&D Company), ice Incubate for 30 minutes; wash the cells with 4ml 1×PBS each time, and wash twice; resuspend the cells in 1ml 1×PBS, ready for detection on the machine.
[0105]After the results of flow cytometry detection (see Figure 4), the screened cell lines Clone1 and Clone2 both expressed the targ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com