Method for synthesizing acetylene alcohol polyoxyethylene ether
A technology of acetylene glycol polyoxyethylene ether and synthesis method, which is applied in the synthesis field of acetylene glycol polyoxyethylene ether and can solve the problem of carbon-carbon triple bond destruction, loss of unsaturation of acetylene glycol polyoxyethylene ether, acetylene di The problem of high degree of unsaturation of alcohol polyoxyethylene ether
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
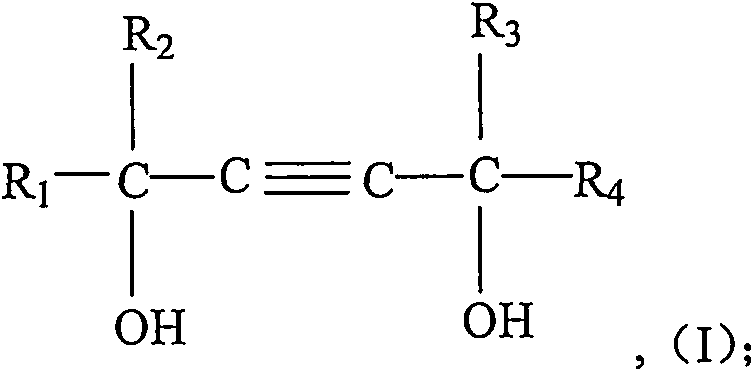
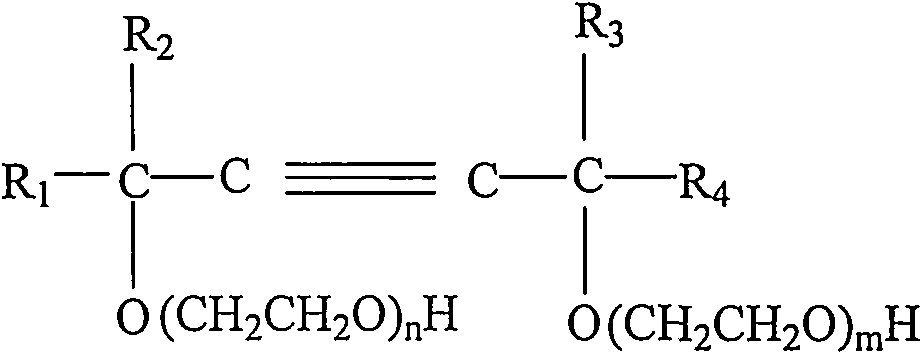
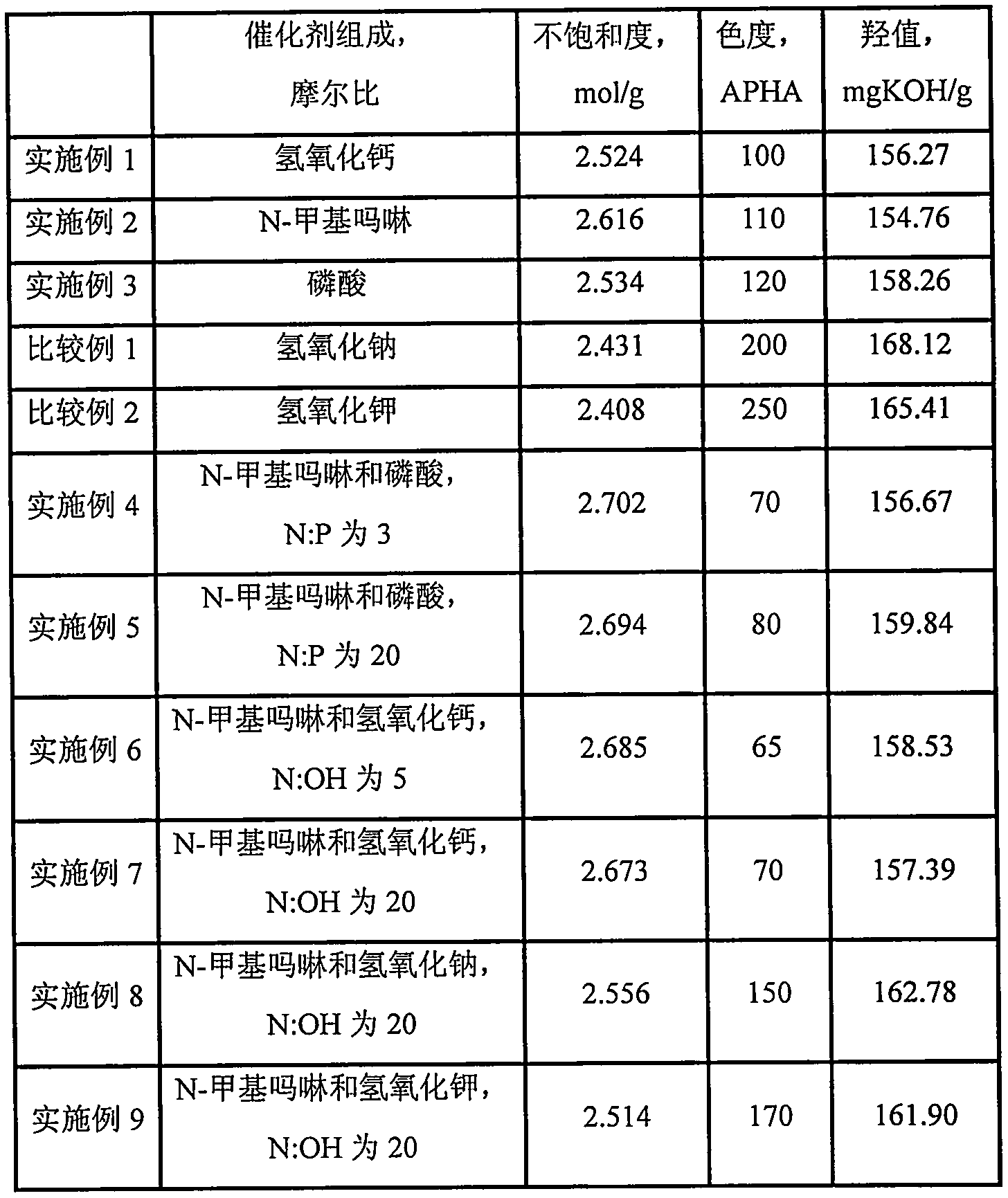
[0032] In a 1L stirred autoclave, add 1 mole of 2,4,7,9-tetramethyl-5-decyne-4,7-diol and 5 grams of catalyst (catalyst is calcium hydroxide), seal the reactor, Turn on stirring. The air in the reactor was replaced with nitrogen three times, and then vacuumed at 60° C. and a pressure of −0.05 MPa for 30 min. Then feed oxirane in the reactor, control reaction temperature to be 120 ℃ and reaction pressure be 0.2MPa, be 10 moles until the total amount of passing oxirane, then stop feeding oxirane to complete subject response. The temperature of the reactor was maintained at 120° C. until the pressure of the reactor no longer dropped, indicating that the aging reaction was completed. Lower the temperature to 80°C, neutralize with citric acid, dehydrate in vacuum, and filter to obtain the product 2,4,7,9-tetramethyl-5-decyne-4,7-diol polyoxyethylene ether (10).
[0033] Determine the degree of unsaturation, chroma and hydroxyl value of the product. For ease of comparison, the c...
Embodiment 2
[0035] Add 1 mole of 2,4,7,9-tetramethyl-5-decyne-4,7-diol and 5 grams of catalyst (the catalyst is N-methylmorpholine) into a 1L stirred autoclave, seal Reactor, start stirring. The air in the reactor was replaced with nitrogen three times, and then vacuumed at 60° C. and a pressure of −0.05 MPa for 30 min. Then feed oxirane in the reactor, control reaction temperature to be 120 ℃ and reaction pressure be 0.2MPa, be 10 moles until the total amount of passing oxirane, then stop feeding oxirane to complete subject response. The temperature of the reactor was maintained at 120° C. until the pressure of the reactor no longer dropped, indicating that the aging reaction was completed. Lower the temperature to 80°C, neutralize with citric acid, dehydrate in vacuum, and filter to obtain the product 2,4,7,9-tetramethyl-5-decyne-4,7-diol polyoxyethylene ether (10).
[0036] Determine the degree of unsaturation, chroma and hydroxyl value of the product. For ease of comparison, the c...
Embodiment 3
[0038] Add 1 mole of 2,4,7,9-tetramethyl-5-decyne-4,7-diol and 5 grams of catalyst (the catalyst is H 3 PO 4, with a concentration of 85w% in the form of commercially available concentrated phosphoric acid), seal the reactor, and start stirring. The air in the reactor was replaced with nitrogen three times, and then vacuumed at 60° C. and a pressure of −0.05 MPa for 30 min. Then feed oxirane in the reactor, control reaction temperature to be 120 ℃ and reaction pressure be 0.2MPa, be 10 moles until the total amount of passing oxirane, then stop feeding oxirane to complete subject response. The temperature of the reactor was maintained at 120° C. until the pressure of the reactor no longer dropped, indicating that the aging reaction was completed. Lower the temperature to 80°C, neutralize with ammonia water, dehydrate in vacuum, and filter to obtain the product 2,4,7,9-tetramethyl-5-decyne-4,7-diol polyoxyethylene ether (10).
[0039] Determine the degree of unsaturation, ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com