Method for improving cyclization activity of cyclodextrin glucosyltransferase
A glucose-based and cyclodextrin technology, which is applied in the fields of genetic engineering and enzyme engineering, can solve the problems of low cyclization activity and high industrial production cost of cyclodextrin, and achieve the effect of improving cyclization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
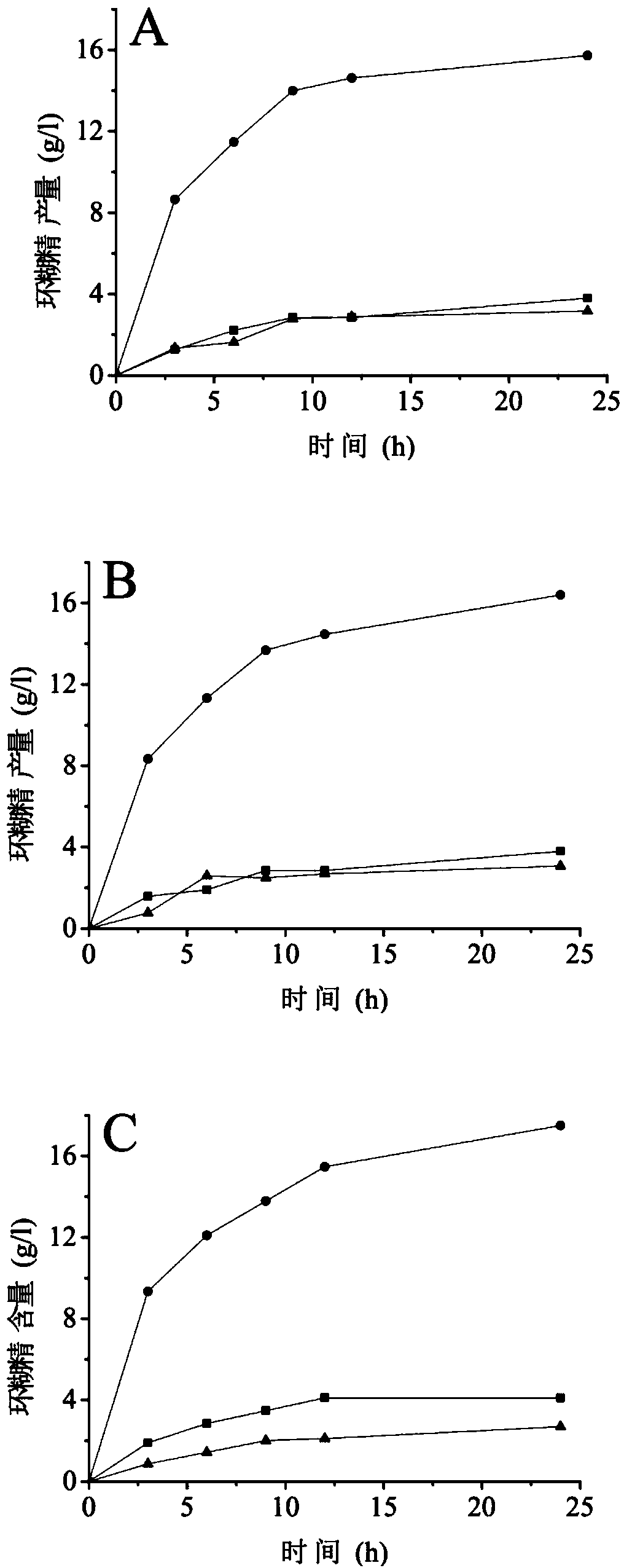
[0032] Example 1 Determination of Mutation Sites
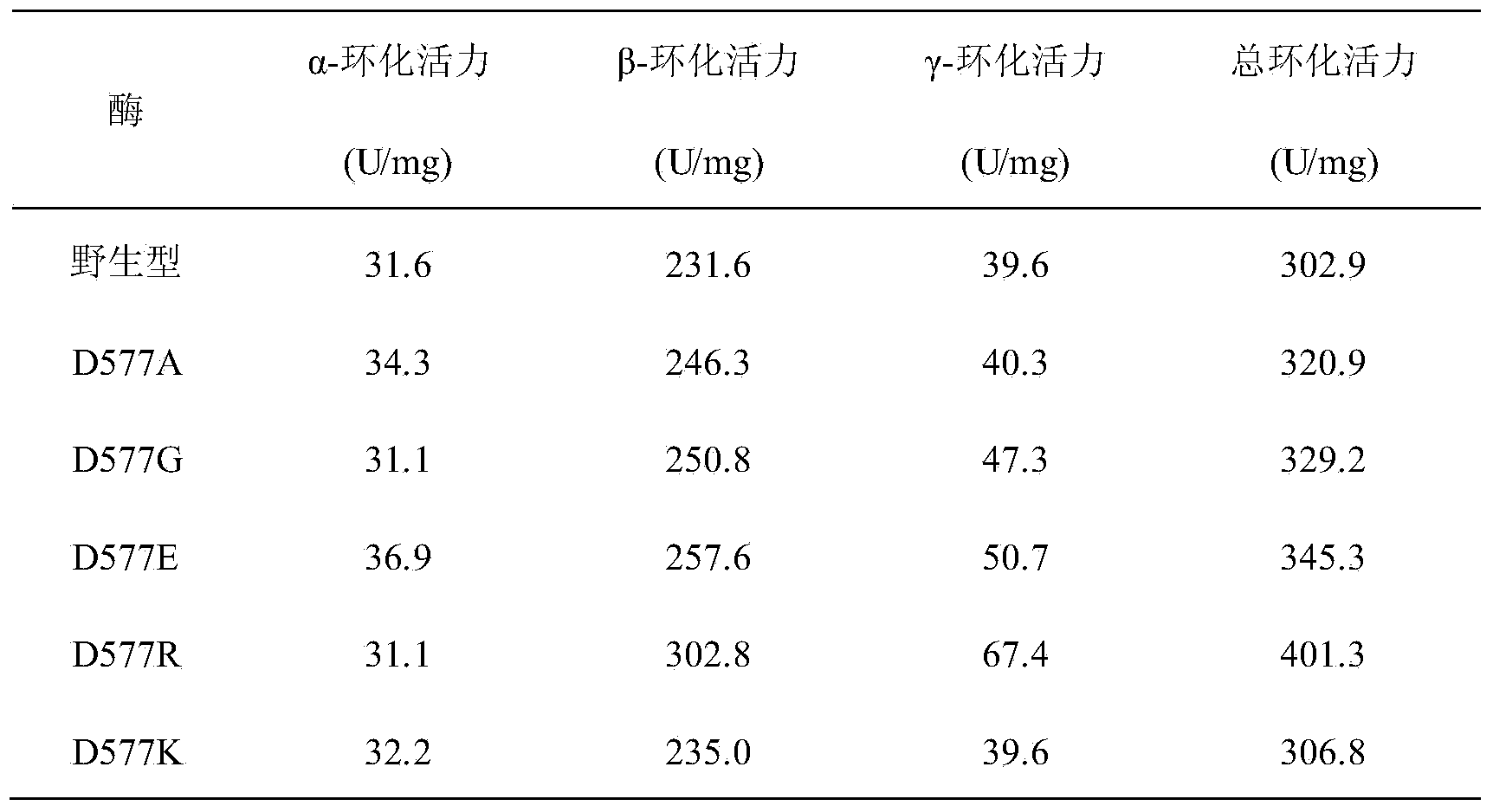
[0033] Calcium ion binding sites widely exist in the α-amylase family, and as a member of the α-amylase family (family 13), CGT enzymes also have similar calcium ion binding sites. Through sequence alignment and crystal structure analysis, it was found that the calcium ion binding site CaⅢ was significantly different in CGTases from different sources, which suggested that the change of amino acid residues at this site may affect the activity of the enzyme. The calcium ion binding site of CGTase derived from Bacillus circulans STB01 consists of two amino acid residues, Ala315 and Asp577.
Embodiment 2
[0034] Preparation of embodiment 2 mutants D577A, D577G, D577E, D577R and D577K
[0035] (1) Site-directed mutation
[0036] According to the wild CGTase gene sequence shown in SEQ ID NO.1, primers for introducing mutations in Ala577, Gly577, Glu577, Arg577 and Lys577 codons were designed and synthesized respectively.
[0037] Using rapid PCR technology, site-directed mutagenesis was performed using the expression vector cgt / pST containing the wild CGTase gene as a template.
[0038] Primers for site-directed mutagenesis introducing the Ala577 codon:
[0039] Forward primer: 5'-GCAATGTGTAT GCT AACTTCGAG-3', the underline is the mutant base,
[0040] Reverse primer: 5'-CTCGAAGTT AGC ATACACATTGC-3', the underline is the mutant base;
[0041] Primers for site-directed mutagenesis introducing the Gly577 codon:
[0042] Forward primer: 5'-GCAATGTGTAT GGT AACTTCGAG-3', the underline is the mutant base,
[0043] Reverse primer: 5'-CTCGAAGTT ACC ATACACATTGC-3', the underli...
Embodiment 3
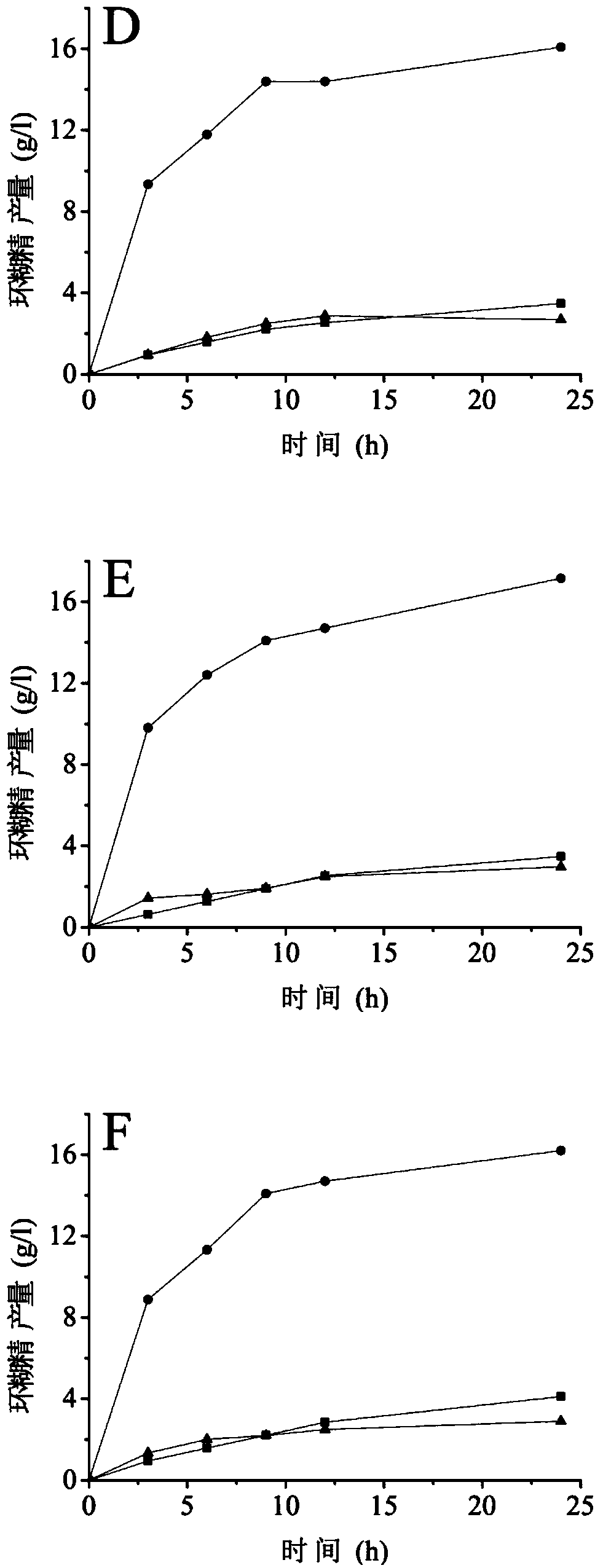
[0059] Embodiment 3 Enzyme assay analysis
[0060] (1) Determination of enzyme activity
[0061] Determination of α-cyclization activity: Take 0.1 mL of appropriately diluted enzyme solution, add 0.9 mL of 1% (w / v) maltodextrin (DE=5) prepared in advance with 10 mM phosphate buffer (pH 6.5) In the test tube of the solution, after reacting at 50°C for 10min, add 1.0mL of 1.0N hydrochloric acid to stop the reaction, then add 1.0mL of 0.1mM methyl orange solution prepared with 10mM phosphate buffer, keep warm at 20°C for 15min, and measure at 505nm Absorbance. Using the inactivated enzyme as a blank, the content of α-cyclodextrin was determined corresponding to the α-cyclodextrin standard curve. One enzyme activity unit is defined as the amount of enzyme required to generate 1 μmol of cyclodextrin per minute under the above conditions.
[0062] Determination of β-cyclization activity: Take 0.1 mL of appropriately diluted enzyme solution, add 0.9 mL of 1% (w / v) maltodextrin (DE...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com