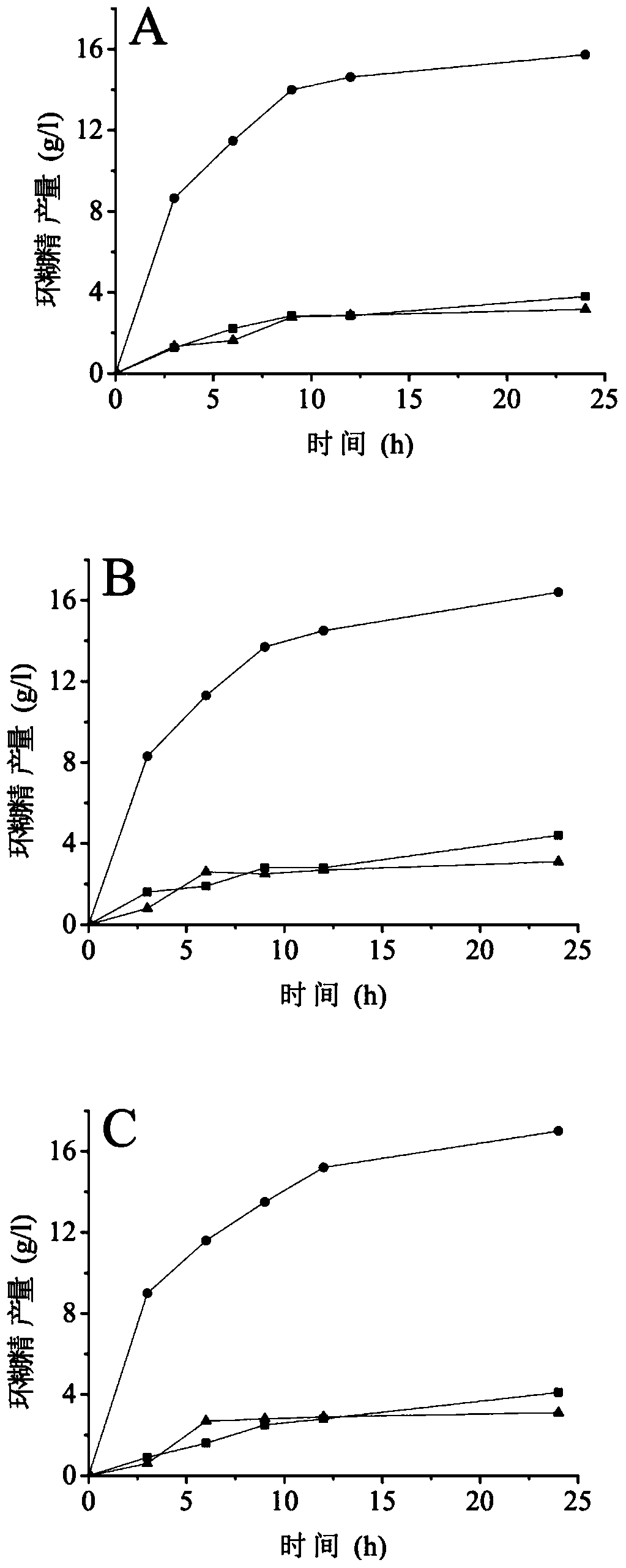
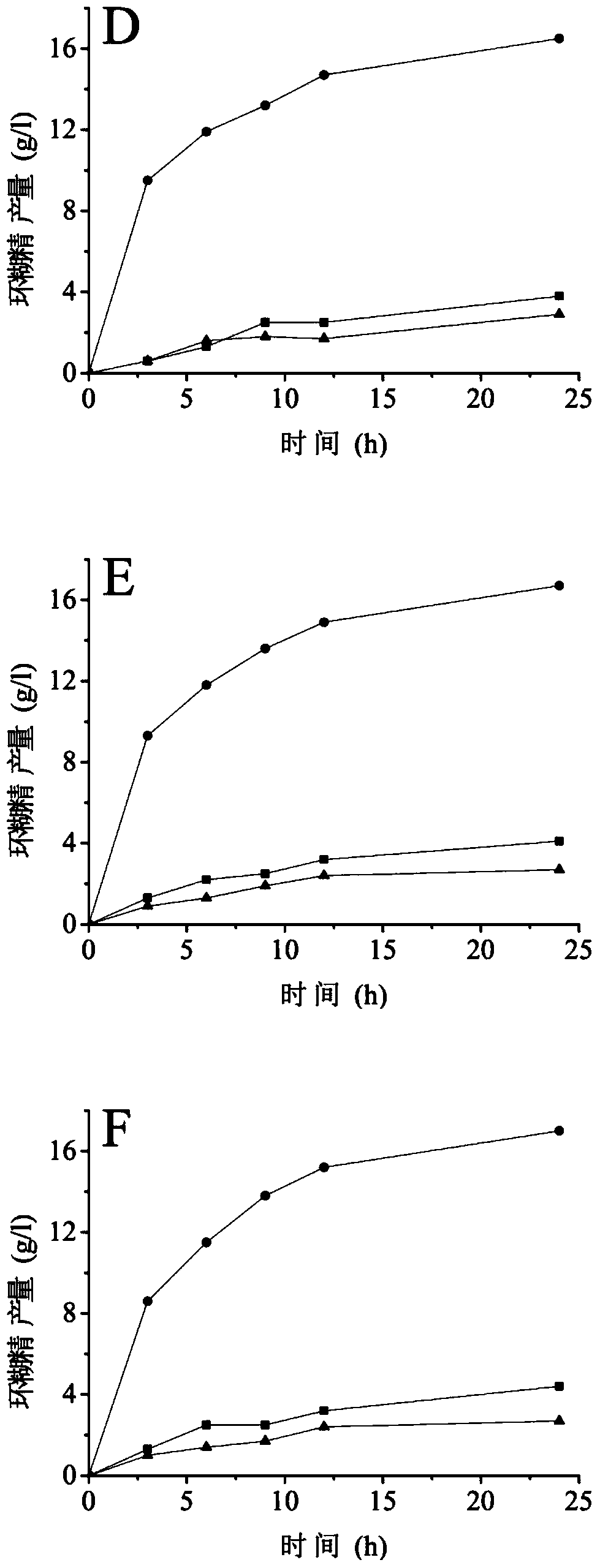
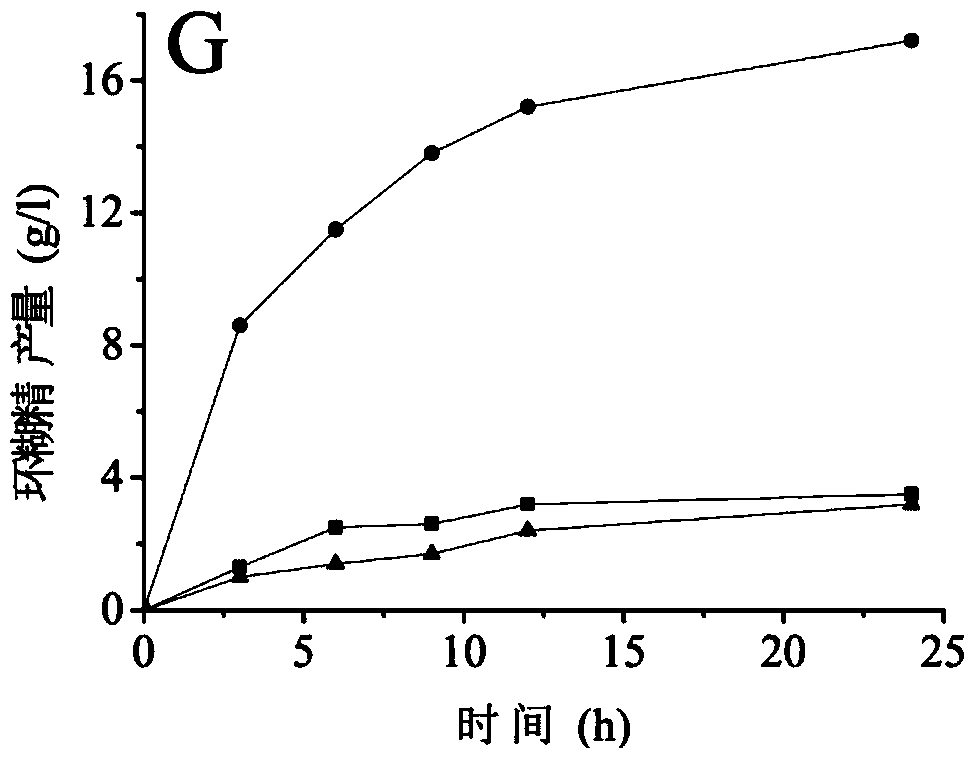
Cyclodextrin glucosyltransferase mutant with improved cyclization activity
A glucose-based, mutant technology, applied in the fields of genetic engineering and enzyme engineering, can solve the problems of low cyclization activity and high production cost of cyclodextrin industry, and achieve the effect of improving cyclization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Determination of Mutation Sites
[0036] Calcium ion binding sites widely exist in the α-amylase family, and as a member of the α-amylase family (family 13), CGT enzymes also have similar calcium ion binding sites. The 32nd amino acid residue asparagine (Asn) of the CGTase derived from Bacillus circulans STB01 is located at the calcium ion binding site CaI, and the spatial structure of the amino acid residues at this site is complex: the distance from Asn32 The polar hydrophilic amino acid residues within are Asp27, Asn29, Asn33, Gly51, Asp53, Tyr112, Gly113, Asp115; The non-polar hydrophobic amino acid residues within are Pro30, 34, 110, Ala31, 111. This diverse and complex conformation suggests that the introduction of longer side chains or more electronegative polar hydrophilic amino acid residues at this site may affect the activity of the enzyme.
Embodiment 2
[0037] Preparation of embodiment 2 mutants N32R, N32K, N32H, N32E, N32D and N32Q
[0038] (1) Site-directed mutation
[0039] According to the wild CGTase gene sequence shown in SEQ ID NO.1, primers for introducing Arg32, Lys32, His32, Glu32 and Asp32 codon mutations were designed and synthesized respectively.
[0040] Using rapid PCR technology, site-directed mutagenesis was performed using the expression vector cgt / pST containing the wild CGTase gene as a template.
[0041] Primers for introducing the Asn32Arg mutation:
[0042] Forward primer: 5'-GCAATCCCGCC AGA AATCCGACC-3', the underline is the mutant base,
[0043] Reverse primer: 5'-GGTCGGATT TCT GGCGGGATTGC-3', the underline is the mutant base;
[0044] Primers for introducing the Asn32Lys mutation:
[0045] Forward primer: 5'-GCAATCCCGCC AAA AATCCGACC-3', the underline is the mutant base,
[0046] Reverse primer: 5'-GGTCGGATT TTT GGCGGGATTGC-3', the underline is the mutant base;
[0047] Primers for introdu...
Embodiment 3
[0065] Embodiment 3 Enzyme assay analysis
[0066] (1) Determination of enzyme activity
[0067] Determination of α-cyclization activity: Take 0.1 mL of appropriately diluted enzyme solution, add 0.9 mL of 1% (w / v) maltodextrin (DE=5) prepared in advance with 10 mM phosphate buffer (pH 6.5) In the test tube of the solution, after reacting at 50°C for 10min, add 1.0mL of 1.0N hydrochloric acid to stop the reaction, then add 1.0mL of 0.1mM methyl orange solution prepared with 10mM phosphate buffer, keep warm at 20°C for 15min, and measure at 505nm Absorbance. Using the inactivated enzyme as a blank, the content of α-cyclodextrin was determined corresponding to the α-cyclodextrin standard curve. One enzyme activity unit is defined as the amount of enzyme required to generate 1 μmol of cyclodextrin per minute under the above conditions.
[0068] Determination of β-cyclization activity: Take 0.1 mL of appropriately diluted enzyme solution, add 0.9 mL of 1% (w / v) maltodextrin (DE...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com