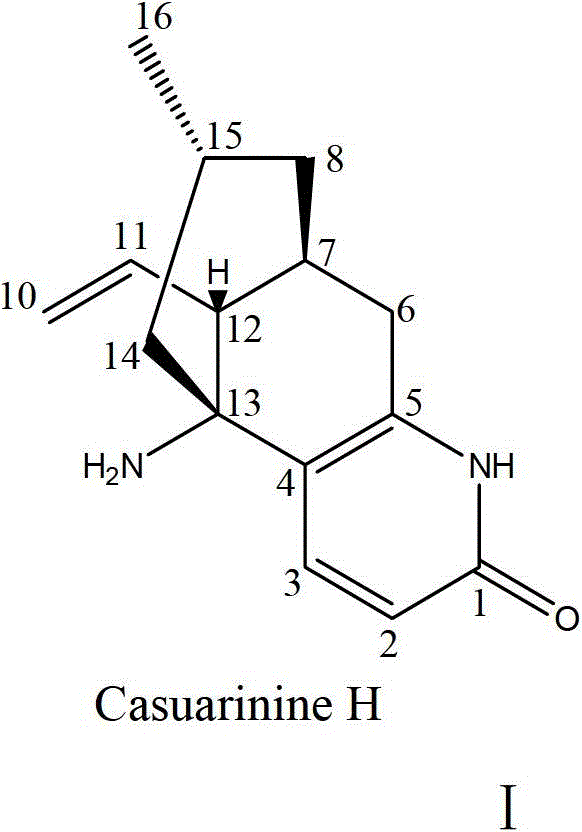
Alkaloid casuarinine H and its use in the preparation of medicines for treating neurodegenerative diseases
A neurodegenerative and alkaloid technology, applied in nervous system diseases, neuromuscular system diseases, drug combinations, etc., can solve problems such as drugs for neurodegenerative diseases that have not yet been seen, and achieve rich natural resources, strong regeneration ability, and wide distribution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1 kg of the whole plant of Fujishipine, crushed, and cold-soaked with 3 times the amount of 90% methanol at room temperature for 24 hours, a total of 3 times. The extracts were combined three times and concentrated under reduced pressure to obtain 90 g of extract. The extract was suspended with 2 times the amount of 3% tartaric acid aqueous solution, extracted three times with ethyl acetate equal to the volume of the suspension, and then washed with Na 2 CO 3 Adjust the tartaric acid aqueous solution to pH = 10, then extract three times with chloroform, and concentrate the chloroform extraction under reduced pressure to obtain 1.1 g of total alkaloid extract, then mix the sample with equal-fold silica gel (100-200 mesh), and use 50 times Silica gel (200-300 mesh) was used for column chromatography, and dichloromethane: methanol was used as the mobile phase gradient elution (volume ratio 1:0-0:1). Collect dichloromethane:methanol (15:1, volume ratio) eluted fractions, ...
Embodiment 2
[0020]1 kg of the whole herb of Fujishipine was crushed, refluxed with 80% ethanol at room temperature for 3 hours, repeated 3 times, the extracts were combined, concentrated under reduced pressure to obtain 110 g of extract. The extract was suspended with 2 times the amount of 3% tartaric acid aqueous solution, extracted three times with ethyl acetate equal to the volume of the suspension, and then washed with Na 2 CO 3 The tartaric acid aqueous solution was adjusted to pH = 10, and extracted three times with an equal volume of chloroform. The chloroform extraction part was concentrated under reduced pressure to obtain 1.3 g of total alkaloid extract. The macroporous resin HP-20 on the extract was eluted with water, 50% ethanol, and 90% ethanol respectively, and the eluted part of 50% ethanol was applied to Sephadex LH-20, which was eluted with dichloromethane:methanol 2:1, and passed TLC detection combined to obtain 13 mg of Casuarinine H.
Embodiment 3
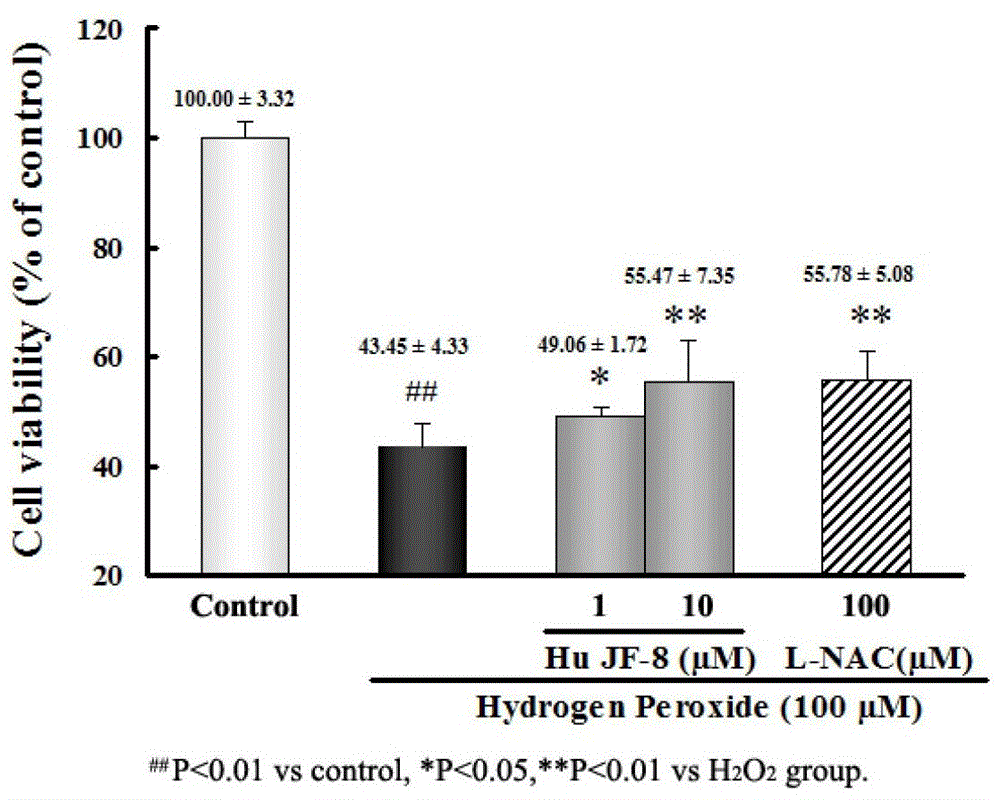
[0021] The neuroprotective effect test of embodiment 3 Casuarinine H
[0022] SHSY-5Y cells were digested and suspended in MEM / F12 medium containing 10% fetal bovine serum. Take 2×10 5 SHSY-5Y cells were seeded on 96-well culture plates at a density of 1 / ml, and the inoculation volume was 100 μl / well, and then placed in 5% CO 2 Incubate in a constant temperature incubator at 37°C for 24 hours. Replace the culture solution in each well with fresh MEM / F12 culture solution, add the corresponding concentration of the compound to be tested (10 microliters / well) in the administration group, normal control group and H 2 o 2 The compound solvent control (10 μl / well) was added to the injury group. After incubation for 2 hours, in the administration group with H 2 o 2 1mM H 2 o 2 (10 μl / well), the final concentration is 100 μM. After continuing to culture for 24 hours, add 5 mg / mL MTT (10 μl / well) for live cell staining. After incubation for 3 hours, discard the culture medium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com