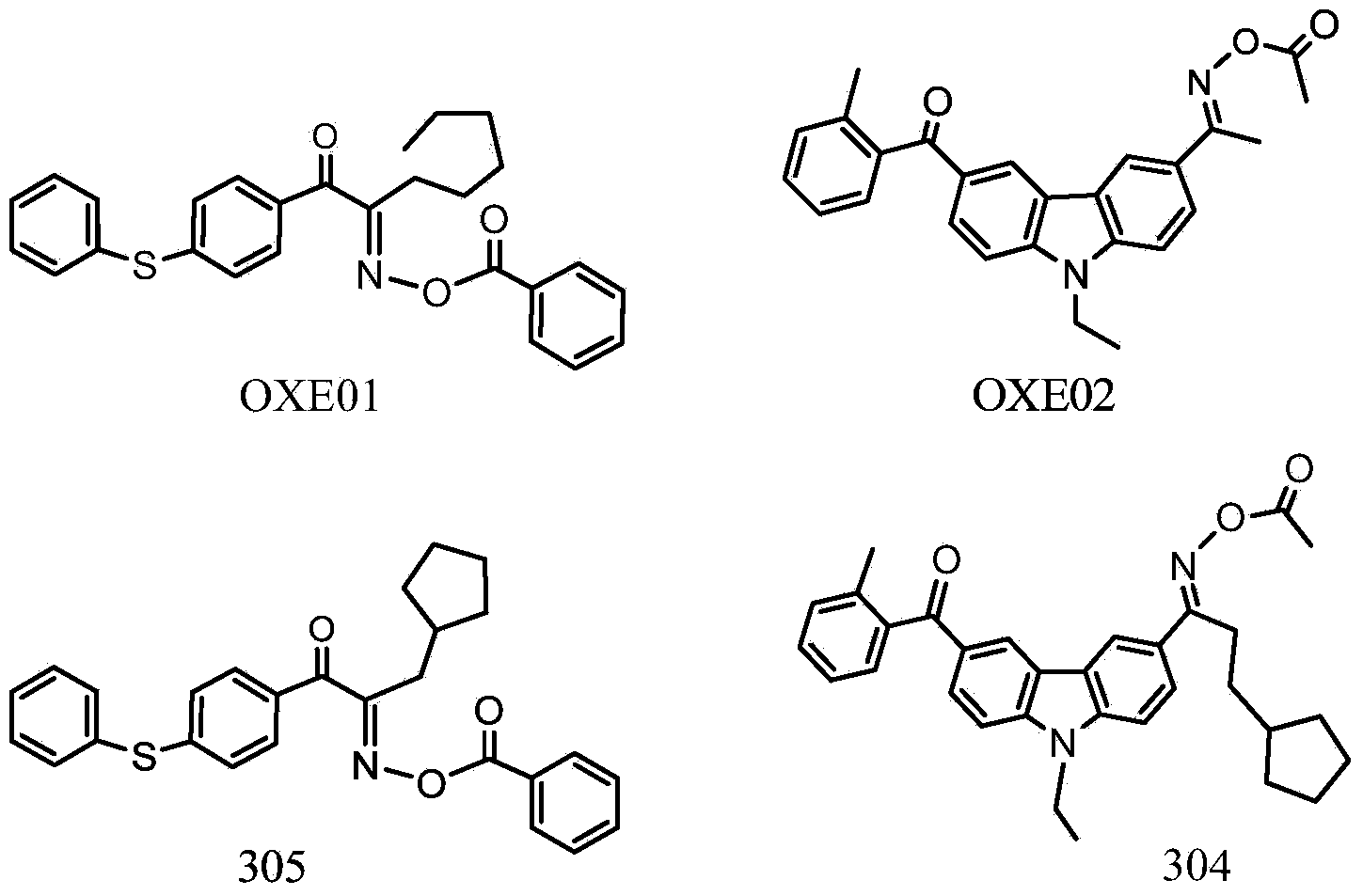
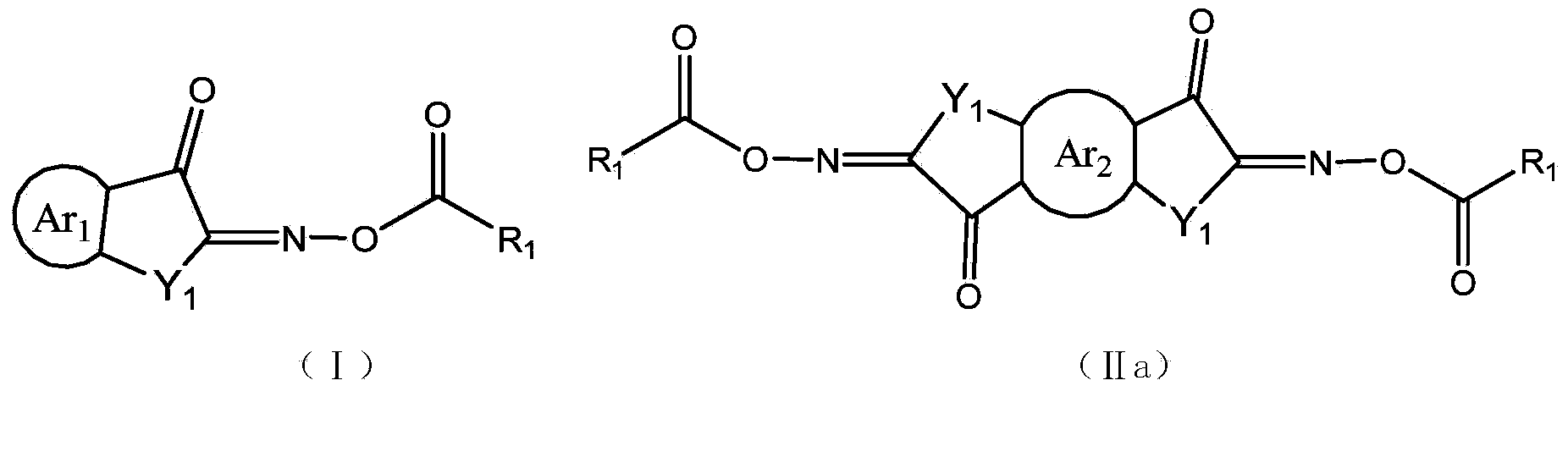
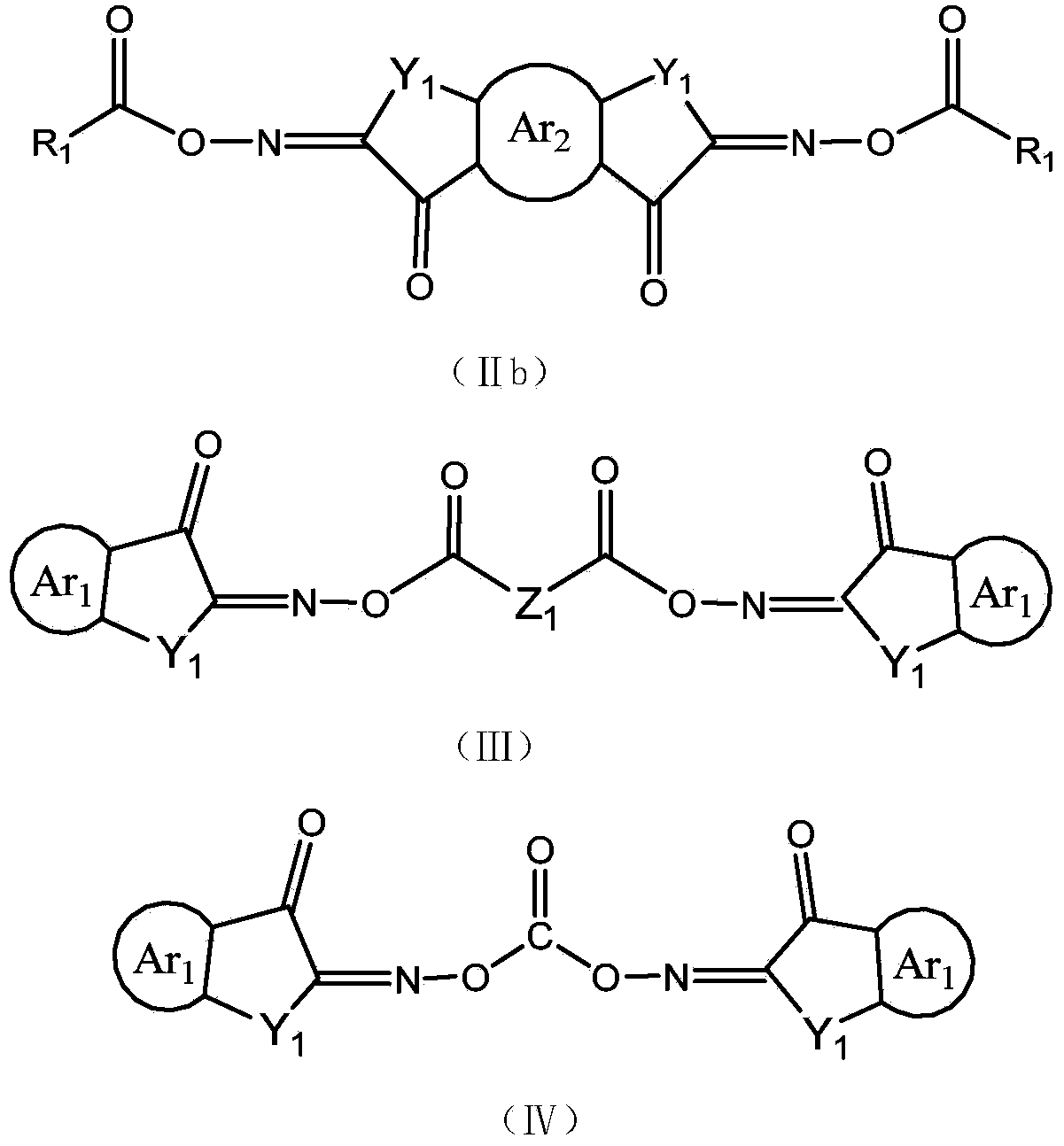
Cyclopentadiketoxime ester and applications thereof
A cycloalkyl and compound technology, applied in the field of oxime ester compounds, can solve the problems of no improvement in photosensitivity, increase in molecular weight, and difficulty in manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Synthesis of 5-methylthioindan-1,2-dione-2-oxime-O-acetate
[0139]
[0140] 1 a , Synthesis of 5-methylthioindan-1-one—etherification method A
[0141]
[0142] Weigh 3.33g (0.02mol) of 5-chloro-1-indanone in a 50ml three-necked flask, add 15ml of N,N-dimethylacetamide, 4.2g (0.03mol) of 50% sodium methyl mercaptide solution, nitrogen Protected, stirred at 20-25°C for 5h, added the reaction solution into 100ml of water, extracted twice with 25ml of 1,2-dichloroethane, combined the 1,2-dichloroethane solution, washed twice with 10ml of water, concentrated the organic solution To dryness, the residue was recrystallized with ethanol, and after drying, 2.5 g of off-white crystals was obtained, with a yield of 70.2%; the analytical purity was 98.02%, and the melting range was 105-106°C.
[0143] 1b, Synthesis of 5-methylthioindan-1,2-dione-2-oxime—oximation method A
[0144]
[0145] Take 1.8g (0.01mol) of the etherification product obtained in 1a, dissolve it i...
Embodiment 2
[0149] Synthesis of 5-(2-Acetoxyethylthio)indan-1,2-dione-2-oxime-O-acetate
[0150]
[0151] 2a, according to the 1a etherification method A of Example 1, with? 2-Mercapto? Ethanol and sodium hydroxide replaced 50% sodium methyl mercaptide solution, reacted with 5-fluoro-1-indanone to synthesize 5-(2-hydroxyethylthio)indan-1-one, light yellow crystal, yield 91.1 %.
[0152]
[0153] 2b, according to the 1b oximation method of Example 1, the product obtained in 2a is? The raw material was oximated and crystallized by methanol to obtain 5-(2-hydroxyethylthio)indan-1,2-dione-2-oxime as a light yellow solid product with a yield of 83.2%.
[0154]
[0155] 2c, according to the 1c esterification method of Example 1, increase the reaction temperature to 45-50 ° C, and obtain the light yellow powdery diacetate product 5-(2-acetoxyethylthio)indan-1 after purification, 2-diketo-2-oxime-O-acetate, purity 99.23%, yield 85.2%, melting range and 1 H-NMR (CDCl 3 ), δ (ppm) va...
Embodiment 3
[0157] Synthesis of 5-(4-isopropylphenylthio)indan-1,2-dione-2-oxime-O-acetate
[0158]
[0159] 3a, Synthesis of 5-(4-isopropylphenylthio)indan-1-one—etherification method B
[0160]
[0161] Weigh 3.33g (0.02mol) of 5-chloro-1-indanone in a 50ml three-necked flask, add 30ml of N,N-dimethylformamide (DMF), 4.2g (0.027mol) of 4-isopropylthiophenol , 3.0g anhydrous sodium carbonate, under nitrogen protection, stirred at 40-45°C for 6h; recovered DMF under reduced pressure, added the residue to 100ml water, extracted twice with 25ml 1,2-dichloroethane, combined 1,2-dichloroethane The ethyl chloride solution was washed twice with 10ml of water, and a 3cm-thick silica gel was spread in a Buchner funnel, and the organic phase was filtered with suction, the filtrate was concentrated to dryness, the residue was recrystallized with methanol, and 5.11g of light yellow crystals were obtained after drying. The yield is 90.6%; the analytical purity is 98.2%, and the melting range i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com