A kind of preparation method of 2-chloroethoxy-2-ethoxydiethanol
A technology of ethoxydiethanol and chloroethoxy is applied in the preparation of ether from alkylene oxide, ether preparation, organic chemistry, etc., and can solve the problems of poor selectivity of the preparation method, unfavorable industrial production, and difficult preparation, and achieve technological progress. Significant, three-waste treatment cost reduction and pollution reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
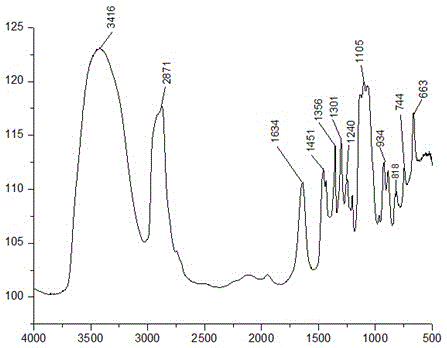
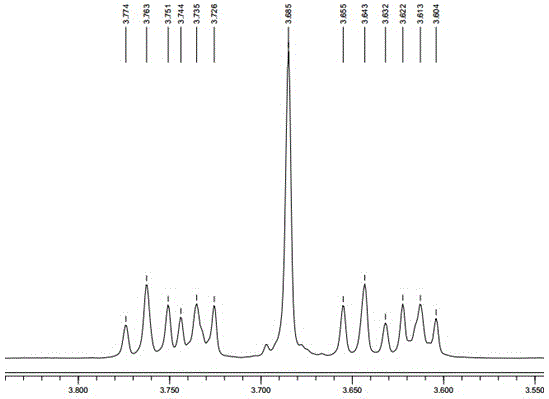
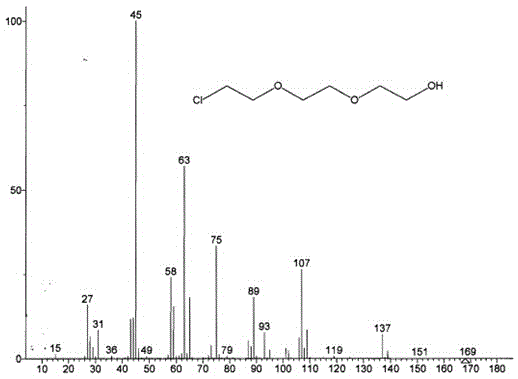
Image
Examples
Embodiment 1
[0038] In a 1000ml three-necked flask, add 685g (8.5mol) of 2-chloroethanol, 3.3g of catalyst, start the stirrer and heater, and raise the temperature to 40°C (in order to maintain the reaction temperature at 40-50°C, the ethylene oxide must be controlled Introduce speed, if necessary, use a water bath to control the temperature), slowly inject 44g of ethylene oxide into the flask, and the introductory time is 1.5-2h. In order to make the reaction complete, after passing through ethylene oxide, continue to stir the reaction for 0.5h. After stirring, heat up, remove excess 2-chloroethanol under reduced pressure, vacuum degree 0.075mPa (water flushing pump), final desolvation temperature 105°C, collect about 592g of 2-chloroethanol (can be recycled), flask The material mainly based on the intermediate product 2-chloroethoxyethanol is obtained.
[0039] Cool the material in the three-necked flask to 45-50°C, add 0.7g of boron trifluoride ether, and pass in 46.2g (1.05mol) of eth...
Embodiment 2
[0042] In a 1000ml three-necked flask, add 685g of 2-chloroethanol (592g+93g new for recycling) (8.5mol), 3.3g of catalyst, start the stirrer and heater, and heat up to 40°C (in order to keep the reaction temperature at 40-50 ℃, to control the speed of ethylene oxide feeding, if necessary, use a water bath to control the temperature), slowly feed 44g of ethylene oxide into the flask, and the feeding time is 1.5-2h. In order to make the reaction complete, after passing through ethylene oxide, continue to stir the reaction for 0.5h. After the stirring is over, start to heat up, remove excess 2-chloroethanol under reduced pressure, the vacuum degree is 0.075mPa (water flushing pump), the final desolvation temperature is 105°C, and about 587g of 2-chloroethanol is collected, which can be used mechanically. Inside is the material mainly containing the intermediate product 2-chloroethoxyethanol.
[0043] Cool the material in the three-necked flask to 45-50°C, add 0.7g of boron trif...
Embodiment 3
[0045] The amount of catalyst used in Example 2 was reduced to 0.3% of the total amount of materials, and the feeding ratio and reaction conditions were the same as in Example 2. After rectification, 116.7 g of the product was obtained, with a gas phase content of 97.6% and a total yield of 69.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com