Preparation method of antigen-specific cytotoxicity T lymphocytes
A lymphocyte and cytotoxic technology, applied in the field of preparation of multi-antigen broad-spectrum CTL, can solve problems such as limited use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Taking targeting carcinoembryonic antigen (CEA) as an example, a CEA-specific gene fragment was obtained through in vitro synthesis and molecular cloning methods. The nucleotide sequence of the carcinoembryonic antigen gene fragment is shown in SEQ ID NO:1; the nucleotide sequence of the melanoma-associated antigen A3 gene fragment is shown in SEQ ID NO:2; the mucin 1 antigen gene fragment is shown in SEQ ID NO : As shown in 3.
Embodiment 2
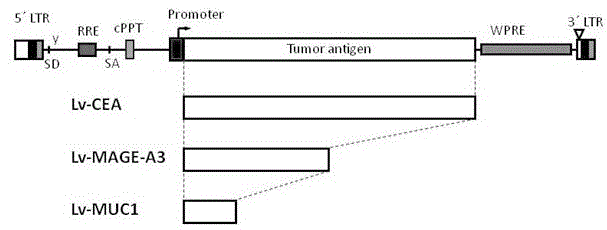
[0029] The gene fragment of described CEA is introduced into lentiviral vector Lv, such as figure 1 The lentiviral vector Lv-CEA carrying the CEA antigen gene was obtained as shown. The same method was used to construct lentiviral vectors with MAGE-A3 and MUC1 antigen gene fragments. Such as figure 1 As shown, among them, the Lv-CEA lentiviral vector is the expression vector carrying the CEA antigen fragment; the Lv-MAGE-A3 lentiviral vector is the expression vector carrying the MAGE-A3 antigen fragment; the Lv-MUC1 lentiviral vector is the expression vector carrying the MUC1 antigen fragment expression vector.
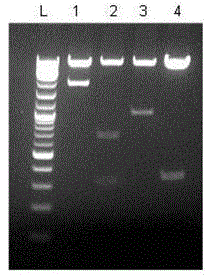
[0030] The lentiviral vectors carrying different chimeric antigen receptor gene fragments were identified by single / double digestion, and the results were as follows: figure 2 and shown in Table 1.
[0031] Table 1 Identification results of lentiviral vectors carrying different tumor antigen gene fragments by enzyme digestion
[0032] carrier name ...
Embodiment 3
[0034] The lentiviral vectors constructed above carrying different tumor antigen genes were transduced into Hela cells. After 48 hours, the cells were lysed, and the expression of each antigen tag protein was detected by Western blot method. The results are as follows: image 3 shown. The results showed that Hela cells themselves did not express CEA, MAGE-A3 and MUC1. After transduction with recombinant vectors, recombinant tumor antigen proteins of the expected size could be detected, indicating that tumor antigen proteins could be expressed in Hela cells after transduction with recombinant vectors. Express correctly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com