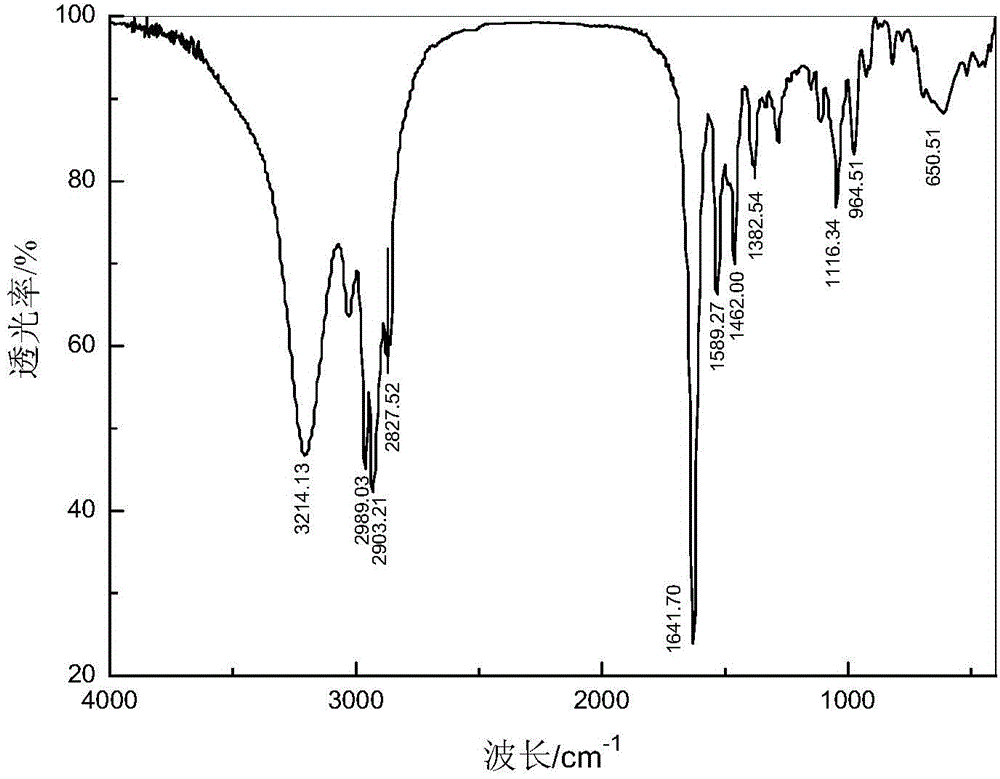
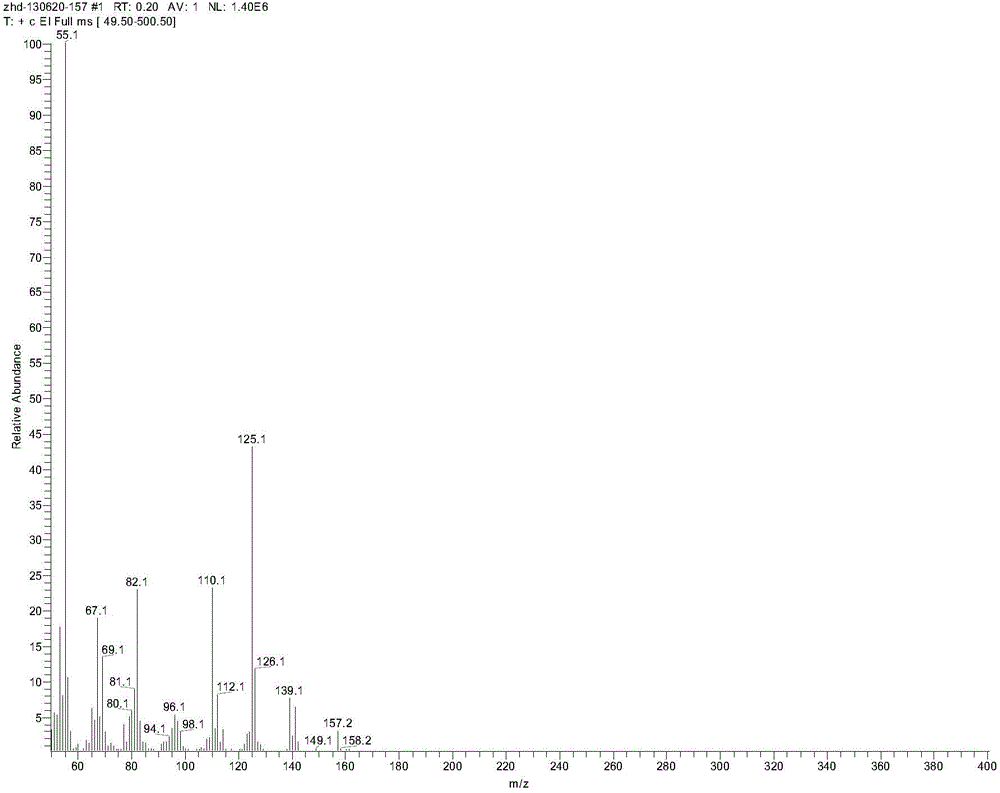
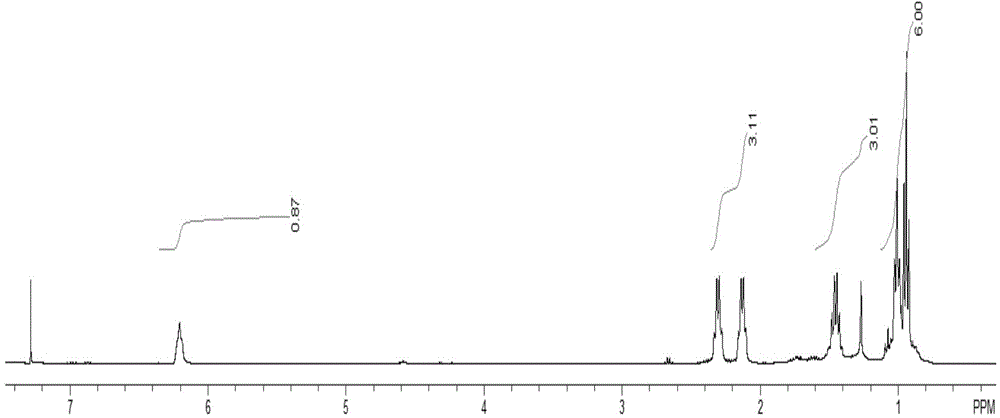
2-ethyl-2-hexenyl hydroximic acid as well as combined collector thereof and application of 2-ethyl-2-hexenyl hydroximic acid and combined collector
A technology of hexenyl hydroxamic acid and combined collectors, applied in solid separation, organic chemistry, flotation, etc., can solve the problems of limited popularization and application, weak collection capacity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 Preparation of alkenyl hydroxamic acid combination collector 1 and 2-ethyl-2-hexenyl hydroxamic acid
[0041] Add 7.1 parts of 2-ethyl-2-hexenoic acid, 9.6 parts of methanol, and 1.0 parts of concentrated sulfuric acid into a reactor with a stirring device, and react at 80°C for 6 hours to obtain 2-ethyl-2-hexene According to the method of national standard GB / T12717-2007, the esterification rate is 94.32%. Dissolve 3.83 parts of hydroxylamine hydrochloride and 4.4 parts of sodium hydroxide in 50.5 parts of methanol, add 13.8 parts of the 2-ethyl-2-hexenoic acid methyl ester product prepared by the previous step reaction, stir and control the temperature at 50oC, and react 3 hours, distilled to obtain the alkenyl hydroxamic acid combined collector, wherein the mass percentage of 2-ethyl-2-hexenyl hydroxamic acid is 71.2%. The 2-ethyl-2-hexenyl hydroxamic acid obtained by the separation and purification of the combined collector through silica gel column ch...
Embodiment 2
[0042] The preparation of embodiment 2 alkenyl hydroxamic acid combined collector 2
[0043] Add 7.1 parts of 2-ethyl-2-hexenoic acid, 9.6 parts of methanol, and 1.0 parts of dodecylbenzenesulfonic acid into a reactor with a stirring device, and react at 80°C for 6 hours to obtain 2-ethyl -2-hexenyl methyl ester product, the esterification rate is 92.58%. Dissolve 3.83 parts of hydroxylamine hydrochloride and 4.4 parts of sodium hydroxide in 45.8 parts of ethanol, add 13.8 parts of 2-ethyl-2-hexenoic acid methyl ester product prepared by the previous step reaction, stir and control the temperature at 50oC, reaction 3 Hours, distilled to obtain alkenyl hydroxamic acid combined collector, wherein the mass percentage of 2-ethyl-2-hexenyl hydroxamic acid is 73.5%.
Embodiment 3
[0044] The preparation of embodiment 3 alkenyl hydroxamic acid combined collector 3
[0045] Add 7.1 parts of 2-ethyl-2-hexenoic acid, 8 parts of methanol, and 1.0 parts of concentrated sulfuric acid into a reactor with a stirring device, and react at 80°C for 6 hours to obtain 2-ethyl-2-hexene Methyl ester product, the esterification rate is 90.65%. Dissolve 3.83 parts of hydroxylamine hydrochloride and 4.4 parts of sodium hydroxide in 48 parts of ethanol, add 13.8 parts of the 2-ethyl-2-hexenoic acid methyl ester product prepared in the previous step reaction, stir and control the temperature at 50oC, and react for 3.5 hours , Distilled to obtain the alkenyl hydroxamic acid combined collector, wherein the mass percentage of 2-ethyl-2-hexenyl hydroxamic acid is 71.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com