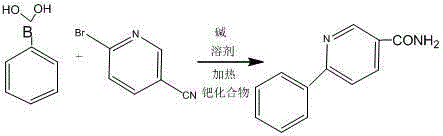
A kind of preparation method of amide compound
A technology of amide compounds and compounds, which is applied in the field of organic compound preparation, can solve problems such as difficult control of reaction progress, and achieve the effects of simple and controllable preparation process, short synthetic route, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 2-bromo-5-cyanopyridine (10mmol, 1.83g), 2,4-difluorophenylboronic acid (10mmol, 1.58g), Na 2 CO 3 (0.5mmol, 57mg), tetrakistriphenylphosphopalladium (0.1mmol, 115mg) was dissolved in a mixed solvent of toluene / ethanol / water (V:V:V=5:2:1), and after reflux reaction for 24 hours, Stop the reaction, let stand at room temperature, wash with dichloromethane, collect the organic phase, and then use silica gel column chromatography to purify and separate to obtain a white solid 2-(2',4'-difluorophenyl)-5- Aminopyridine 2.1g (yield: 89%); Ms (M / Z): 234.1 (M + ); 1 HNMR (DMSO- d 6 ,400MHz), δppm:9.15(s,1H),8.33(dd,J=8Hz,2Hz,1H),8.22(s,1H),8.06(m,1H),7.88(m,1H),7.66(s ,1H),7.44(m,1H),7.27(m,1H).
Embodiment 2
[0031] 2-bromo-5-cyanopyridine (1mmol, 0.183g), phenylboronic acid (1mmol, 0.122g), K 2 CO 3 (0.1mmol, 13.8mg), tetrakistriphenylphosphopalladium (0.01mmol, 11.5mg) was dissolved in a mixed solvent of tetrahydrofuran / water (V:V=3:1), and after reflux reaction for 12 hours, the reaction was stopped and static After placing at room temperature, after washing with dichloromethane, the organic phase was collected, and then purified and separated by silica gel column chromatography to obtain 0.12 g of white solid 2-phenyl-5-amidopyridine (yield: 60%); Ms (M / Z):198.2(M + ).
Embodiment 3
[0033] 2-bromo-5-cyanopyridine (1mmol, 0.183g), 3-methoxy-phenylboronic acid (1mmol, 0.152g), Na 2 CO 3 (0.1mmol, 11.4mg), tetrakistriphenylphosphopalladium (0.1mmol, 11.5mg) was dissolved in a mixed solvent of toluene / water (V:V=2:1), and after reflux reaction for 36 hours, the reaction was stopped and static After placing at room temperature and washing with dichloromethane, the organic phase was collected, purified and separated by silica gel column chromatography to obtain 0.19 g of white solid 2-(3'-methoxyphenyl)-5-amidopyridine ( Yield: 83%); Ms(M / Z): 228.0(M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com