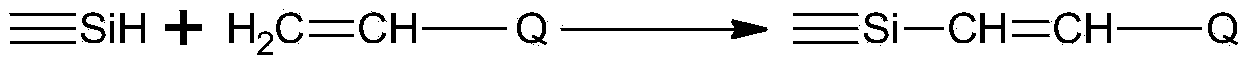Method for synthesizing epoxy modified organosiloxane
A technology for organosiloxane and epoxy modification, which is applied in the field of synthesizing epoxy-modified organosiloxane, which can solve problems such as loss of activity, reduce inconsistency, improve stability and storage, and facilitate reaction conditions The effect of control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
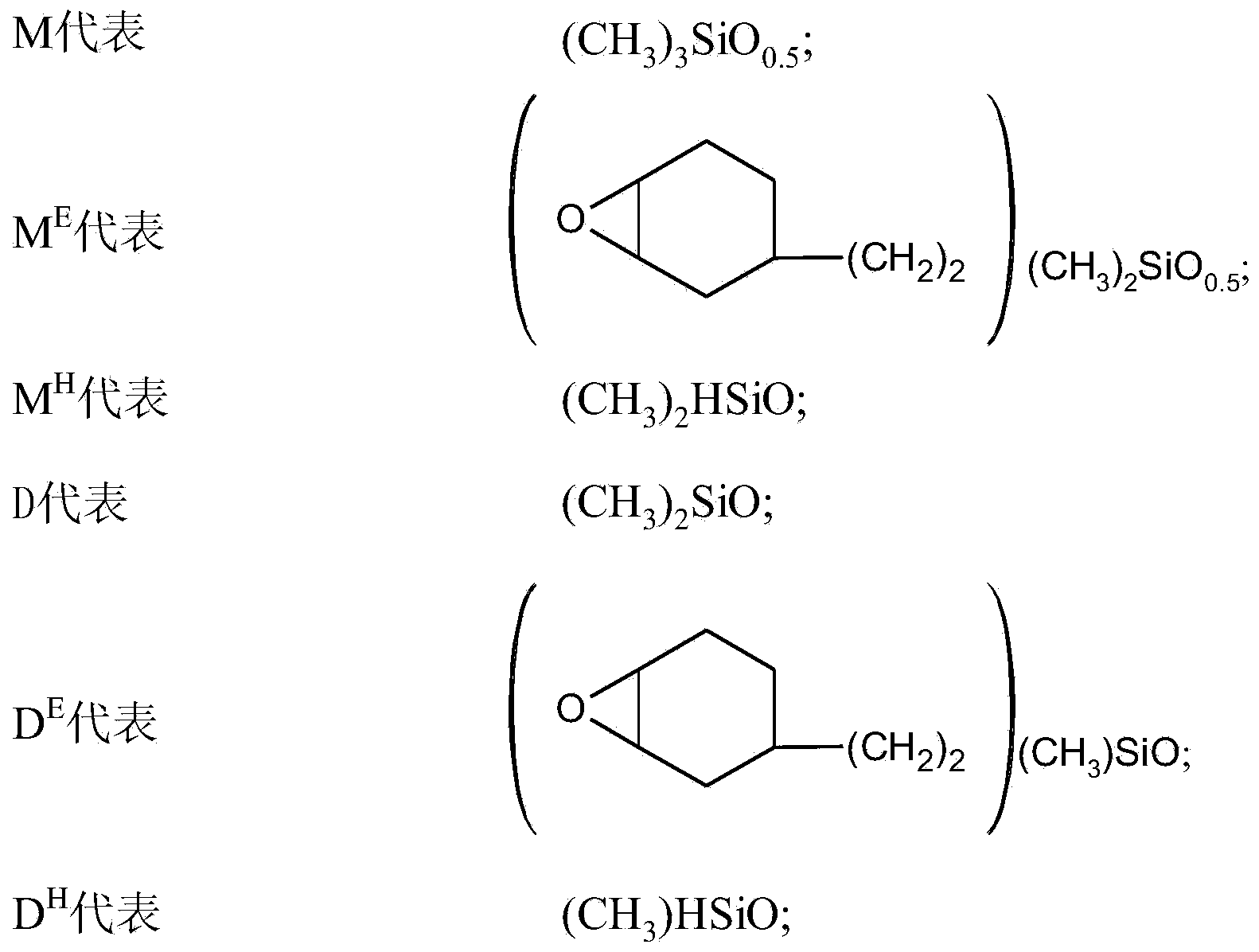
[0031] Add 3g of 1,2-epoxy-4-vinylcyclohexane to a 100ml three-necked flask equipped with a mechanical stirring device and a thermometer, add 15ml of toluene as a solvent, add 3g of ethanol as a stabilizer, add 0.17g of chloroplatinic acid- Divinyltetramethyldisiloxane complex catalyst (also known as Karstedt's catalyzer, the mass content of Pt is 2.6%), at reaction temperature is that this mixture is stirred at 80 ℃, uses dropping funnel to drop 15ml toluene and simultaneously 20g structure is MD 80 D. 6 H M mixed solution of polydimethylsiloxane. After 8 hours of reaction at 80° C., the hydrosilylation reaction can be completed. Examination of the product using infrared spectroscopy revealed complete disappearance of the silicon hydrogen peak. The mixture was vacuum distilled at 50° C. for 1 hour to remove light fractions to obtain 21.4 g of an epoxy-modified organosiloxane product. The epoxy equivalent weight of the product was determined to be 1090.
Embodiment 2
[0033] Add 20g of the structure MD to a 100ml three-neck flask equipped with a mechanical stirring device and a thermometer. 80 D. 6 HM polydimethylhydrogen silicone oil, add 15ml toluene as solvent, do not add any lower alcohol as stabilizer, add 0.17g chloroplatinic acid-divinyltetramethyldisiloxane complex catalyst (also known as Karstedt's catalyst), the mixture was stirred at a reaction temperature of 80° C., and a mixed solution of 15 ml of toluene and 3 g of 1,2-epoxy-4-vinylcyclohexane was added dropwise using a dropping funnel. After reacting for 80 minutes, the system gelled immediately and the reaction could not be continued.
Embodiment 3
[0035] Add 9g of 1,2-epoxy-4-vinylcyclohexane and 15ml of toluene to a 100ml three-necked flask equipped with a mechanical stirring device and a thermometer as a solvent, add 3g of ethanol as a stabilizer, add 0.17g of chloroplatinic acid-di Vinyltetramethyldisiloxane complex catalyst (also known as Karstedt's catalyst), the mixture was stirred at a reaction temperature of 70 ° C, while using a dropping funnel to drop 15ml of toluene and 20g of MD 42 D. 12 H M mixed solution of polydimethylsiloxane. After 8 hours at 70°C, the hydrosilylation reaction can be completed. Examination of the product using infrared spectroscopy revealed complete disappearance of the silicon hydrogen peak. The mixture was vacuum distilled at 50° C. for 1 hour to remove light fractions to obtain 22.1 g of an epoxy-modified organosiloxane product. The epoxy equivalent weight of the product was determined to be 473.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com