Synthesis method of 3-(4-hydroxyphenyl)propanamide
A synthetic method, the technology of hydroxyphenyl, applied in the field of synthesis of 3-propanamide, can solve the problems of troublesome handling, high price, unsuitable for mass production, etc., and achieve cost reduction, convenient post-processing, and omission of catalysts and raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
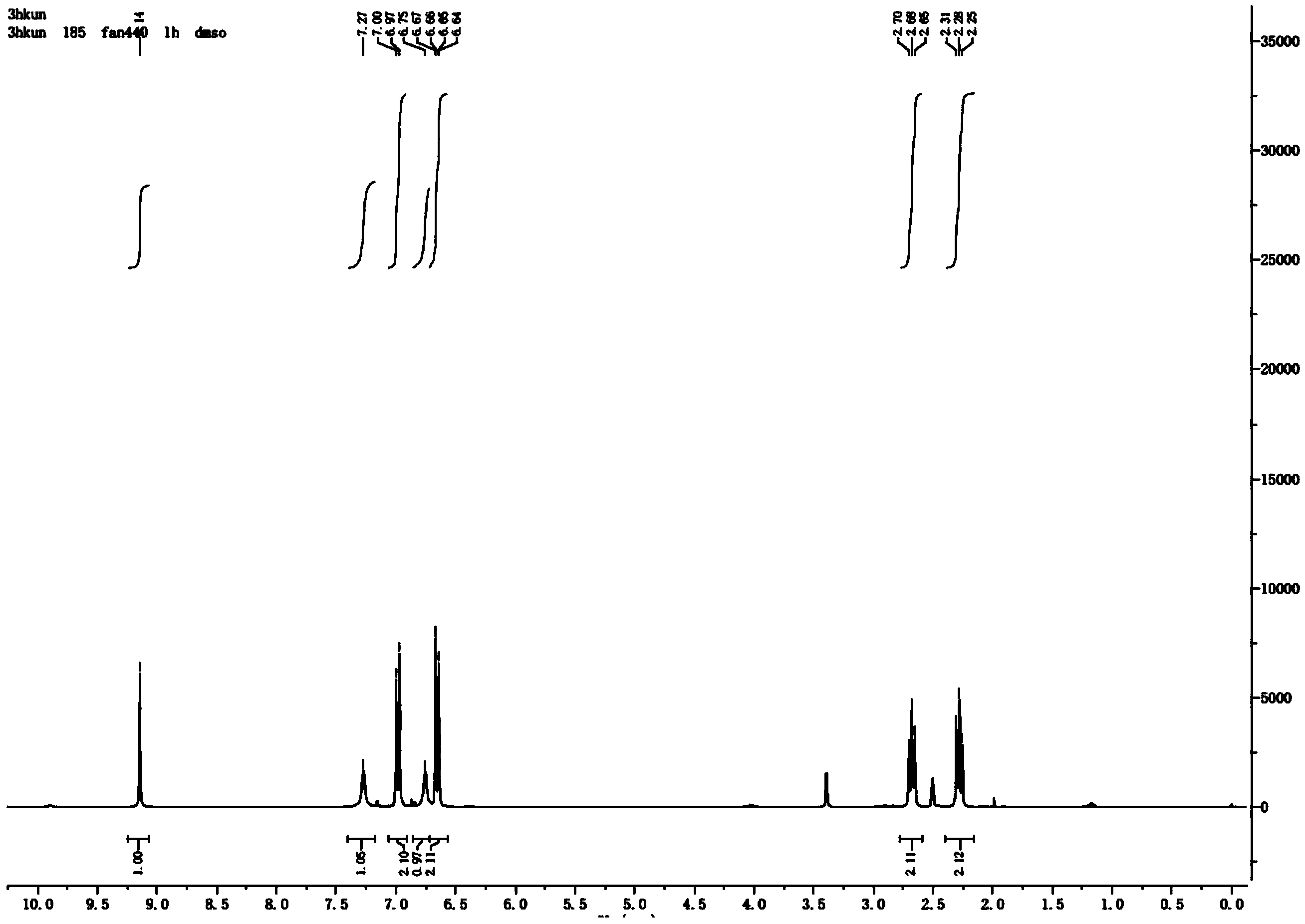
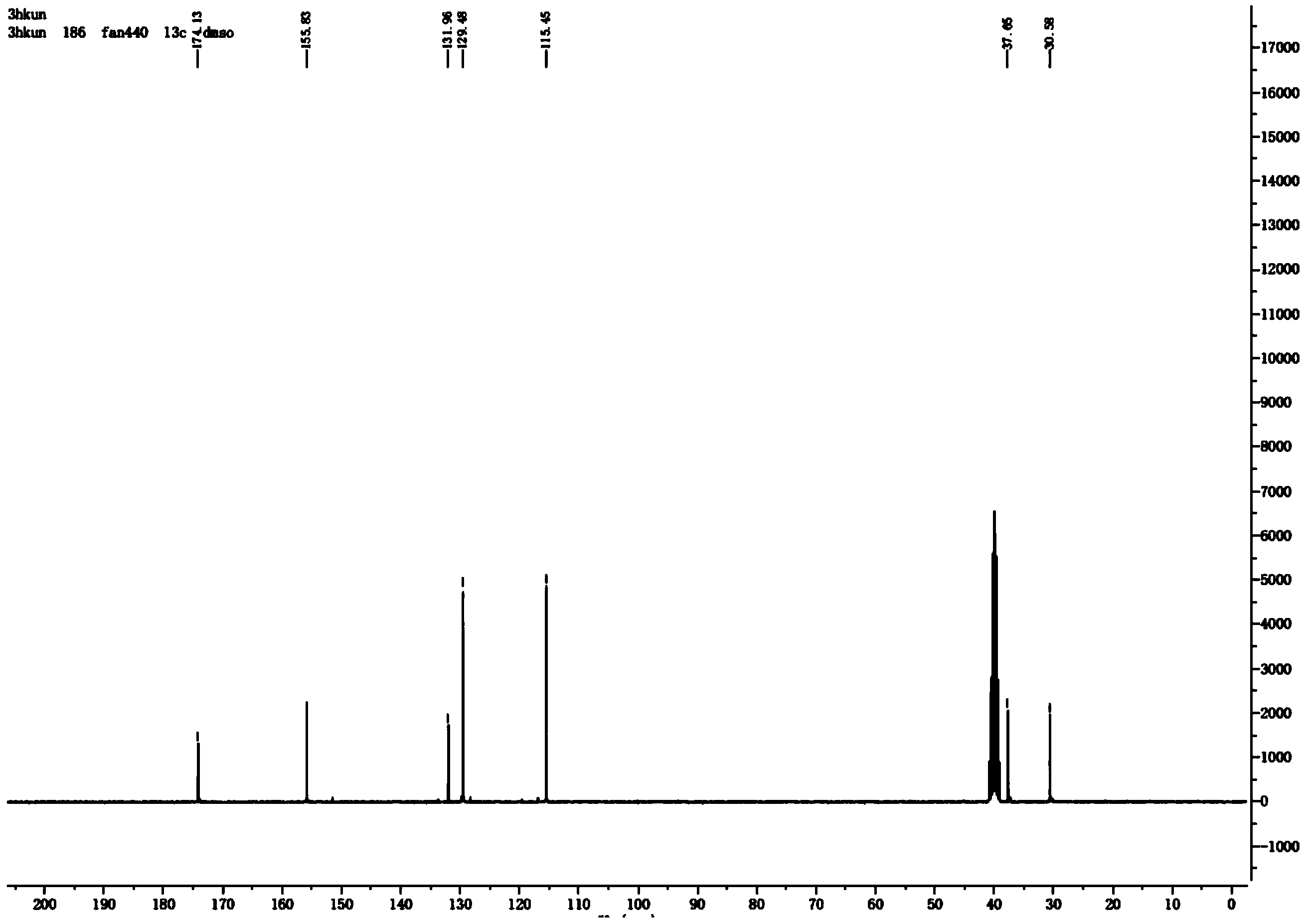
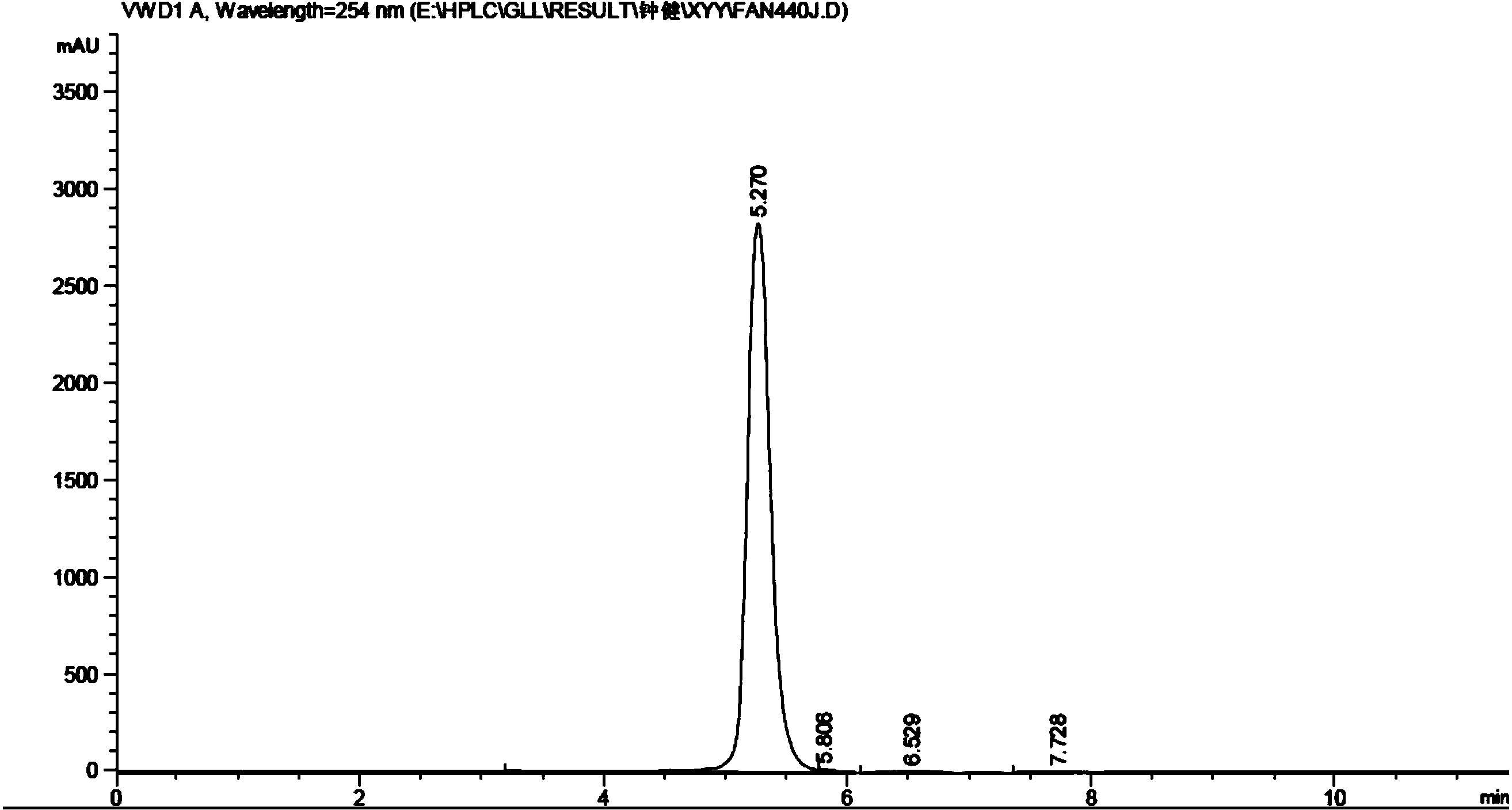
[0024] The synthesis of embodiment 13-(4-hydroxyphenyl) propanamide
[0025] Add 15mL of thionyl chloride to 2g of p-hydroxyphenylpropionamide, stir until clear, take a sample, add a small amount of methanol, PE:EA=5:1 spot plate, about 1-1.5h.
[0026] Then spin off the thionyl chloride, add a small amount of acetonitrile to dissolve, slowly add dropwise to 20mL of 25-28% ammonia water at 0°C, stir for 15-30min, sample, acidify, DCM:MeOH=10:1 plate.
[0027] After the reaction is completed, spin off the solvent, add a small amount of concentrated hydrochloric acid to the obtained solid, the solid turns from yellow to white, spin off the hydrochloric acid, spin dry, add excess ethyl acetate, heat to reflux, dissolve the solid, and filter off the insoluble matter while it is hot , spin off most of the ethyl acetate, put it in ice, and a solid precipitates out. The yield of 3-(4-hydroxyphenyl)propanamide was 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com