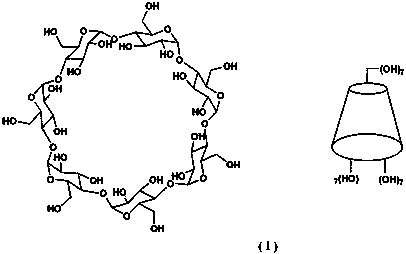
6-site monosubstituted-beta-cyclodextrin functional monomer and preparation method thereof
A technology of functional monomers and cyclodextrins, applied in mono-))-)-6-deoxy)-β-cyclodextrin functional monomers and its preparation, 6-position monosubstituted-β-cyclodextrin functional In the field of monomer and its preparation, it has achieved a wide range of research effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
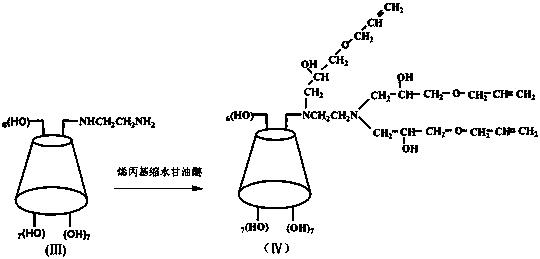
[0035] Embodiment 1: This single-(6-ethylenediamine-( N -(2-bis(3-allyloxy-2-hydroxy-propyl)))-( N -(3-allyloxy-2-hydroxy-propyl))-6-deoxy)-β-cyclodextrin (IV), its structural formula is as follows:
[0036] ;
[0037] Its specific preparation process is as follows:
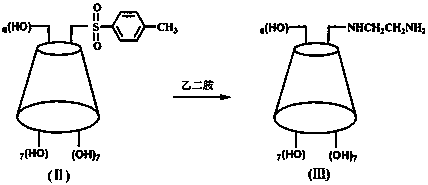
[0038] (1) Synthesis of mono-(6-p-methylbenzenesulfonyl-6-deoxy)-β-cyclodextrin (Ⅱ)
[0039]
[0040] Take 17.22 g (0.0150 mol) of β-cyclodextrin (I) in a 250 mL round bottom flask, add 200 mL of 0.30 mol / L sodium hydroxide solution, stir to dissolve, add dropwise 1.45 g (0.00750 mol) of p-toluenesulfonyl chloride Acetonitrile solution 5.5 mL, dropwise time is 5 min, reaction temperature is 10 °C, after reaction for 2 h, filter after completion of the reaction, adjust pH = 1 with hydrochloric acid, store in refrigerator at 4 °C overnight, a large amount of precipitate precipitates, filter, re- Crystallized to obtain a white solid, which was dried under vacuum at 40°C for 5 h to obtain mono-(6-p-toluenesu...
Embodiment 2
[0047] Embodiment 2: This single-(6-ethylenediamine-( N -(2-bis(3-allyloxy-2-hydroxy-propyl)))-( N -(3-allyloxy-2-hydroxy-propyl))-6-deoxy)-β-cyclodextrin (IV), its structural formula is as follows:
[0048] ;
[0049] Its specific preparation process is as follows:
[0050] (1) Synthesis of mono-(6-p-methylbenzenesulfonyl-6-deoxy)-β-cyclodextrin (Ⅱ)
[0051] Take 17.22 g (0.0150 mol) of β-cyclodextrin (I) in a 250 mL round bottom flask, add 200 mL of 0.25 mol / L sodium hydroxide solution, stir to dissolve, add dropwise 2.90 g (0.0150 mol) of p-toluenesulfonyl chloride The acetonitrile solution was 11 mL, and the dropping time was 10 min. After reacting for 3 h, the reaction temperature was 20°C. After the reaction was completed, it was filtered, adjusted to pH = 2 with hydrochloric acid, and stored in the refrigerator at 4°C overnight. A large amount of precipitate was precipitated, filtered, Recrystallized to obtain a white solid, which was dried in vacuum at 40°C for 5...
Embodiment 3
[0068] Embodiment 3: This single-(6-ethylenediamine-( N -(2-bis(3-allyloxy-2-hydroxy-propyl)))-( N -(3-allyloxy-2-hydroxy-propyl))-6-deoxy)-β-cyclodextrin (IV), its structural formula is as follows:
[0069] ;
[0070] Its specific preparation process is as follows:
[0071] (1) Synthesis of mono-(6-p-methylbenzenesulfonyl-6-deoxy)-β-cyclodextrin (Ⅱ)
[0072] Take 17.22 g (0.0150 mol) of β-cyclodextrin (I) in a 250 mL round bottom flask, add 200 mL of 0.20 mol / L sodium hydroxide solution, stir to dissolve, add dropwise 5.80 g (0.0300 mol) of p-toluenesulfonyl chloride Acetonitrile solution was 20 mL, and the dropwise addition time was 15 min. After reacting for 4 h, the reaction temperature was 30°C. After the reaction was completed, filter, adjust the pH to 3 with hydrochloric acid, and store in the refrigerator at 4°C overnight. Crystallized to obtain a white solid, which was dried in vacuum at 40°C for 5 h to obtain mono-(6-p-toluenesulfonyl-6-deoxy)-β-cyclodextrin (I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com