Method for simultaneously detecting influenza A virus, influenza B virus and influenza C virus and kit
A technology of influenza virus and detection reagents, applied in the field of biotechnology applications, can solve the problems of long time-consuming, difficult detection of trace nucleic acid and accurate quantification, and cumbersome operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1. Design and synthesis of various types of M genes, NS genes and NP gene-specific primers and probes of type A, type B and type C influenza viruses
[0034] 1.1 Selection of corresponding target genes M, NS and NP of representative strains of influenza A, B and C and determination of conserved regions
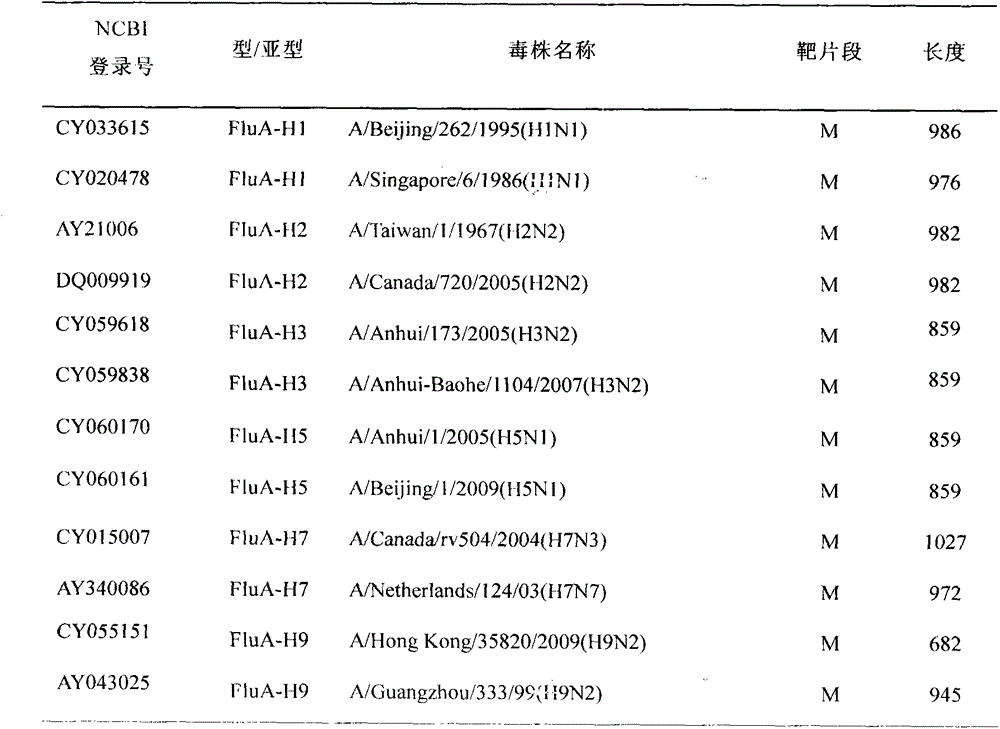
[0035] The sequences used in this study were selected from NCBI GenBank (http: / / www.ncbi.nlm.nih.gov) and the influenza virus database of Los Amos National Laboratory (http: / / www.flu.lanl.gov) Among them, the main basis for selection is: (a) strains with a relatively recent age; (b) strains with full-length sequences and main sequences; (c) vaccine strains recommended by WHO; (d) selection of strains with Representative strains. The information of the corresponding M gene, NS gene and NP gene of selected representative strains of influenza A, B and C are shown in Tables 1-1, 1-2 and 1-3.
[0036] In this study, the corresponding M genes, NS genes and NP gen...
Embodiment 2
[0047] Example 2. Establishment of one-step multiplex fluorescent RT-PCR detection system for influenza A, B and C viruses
[0048] 2.1 According to Invitrogen's nucleic acid extraction kit ( Viral RNA Mini Kit) to extract sample nucleic acid.
[0049] 2.2 Preparation of one-step multiplex fluorescent RT-PCR detection system for influenza A, B and C viruses The AgPath-IDTM One-step RT-PCR Kit from Ambion Company was used for the preparation of reagents, and the system volume was 50 μl:
[0050] 2×RT-PCR buffer 25μl
[0051] 25×RT-PCR Enzyme 2μl
[0052] Detection Enhancer 3μl
[0053] Add 0.5 μl of primers shown in SEQ NO: 1, 2, 4, 5, 7, 8, 10, and 11 at the same time for primers and probes, and the final concentration of primers is 200 nM; add primers such as SEQ NO: 3, 6, 9, 0.5 μl each of the probe primers indicated in 12, the final concentration is 100 nM
[0054] Template 6 μl (add 2 μl for each of the three positive templates, and add each template after 10-1 fold ...
Embodiment 3
[0062] Example 3. Specificity identification of type A, type B and type C influenza virus one-step multiplex fluorescent RT-PCR detection method
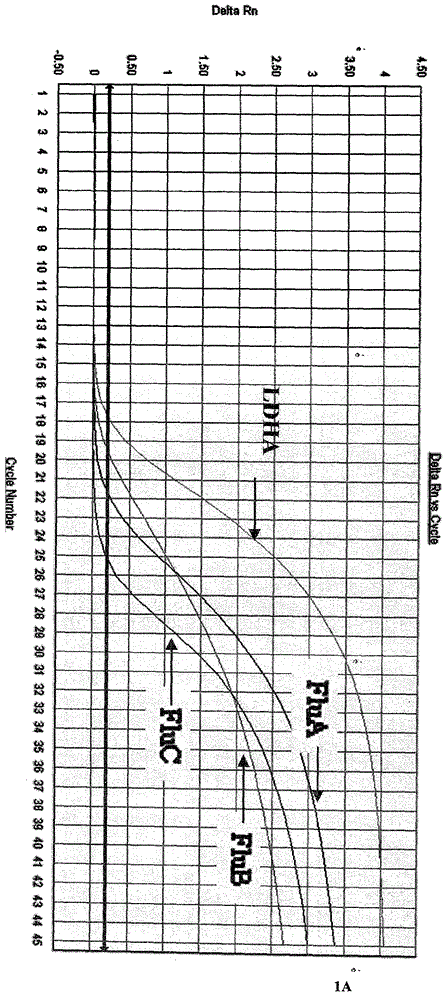
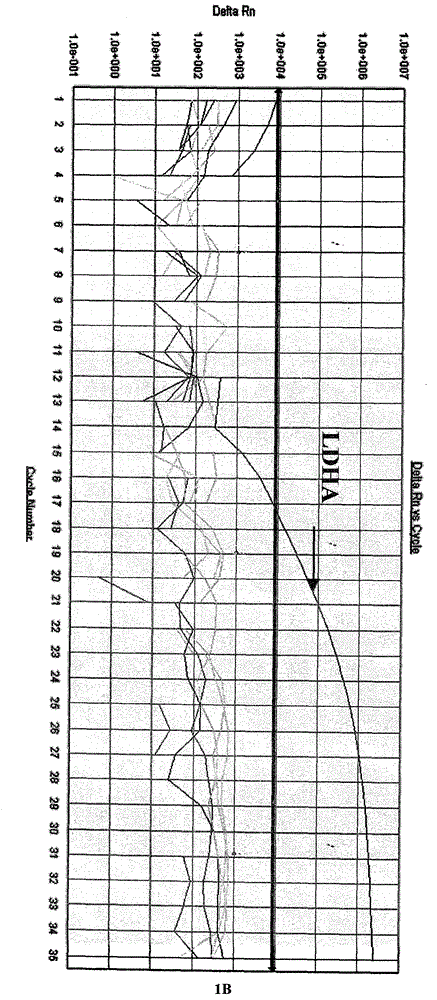
[0063] The one-step multiplex fluorescent RT-PCR reaction system established in Example 2 is used for influenza A virus, influenza B virus, influenza C virus, parainfluenza virus, respiratory syncytial virus, enterovirus, adenovirus, Boca virus, respectively. The positive nucleic acid of the virus was detected, and the results showed that only influenza A, B, and C had corresponding specific fluorescence amplification curves in the FAM, JOE, and ROX detection channels respectively, and there was no cross-reaction, while the other 5 In addition to the amplification curve of the internal quality control CY5 channel of the strain virus, the other three channels have no amplification curve, indicating that the method has strong specificity (see Figure 1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com