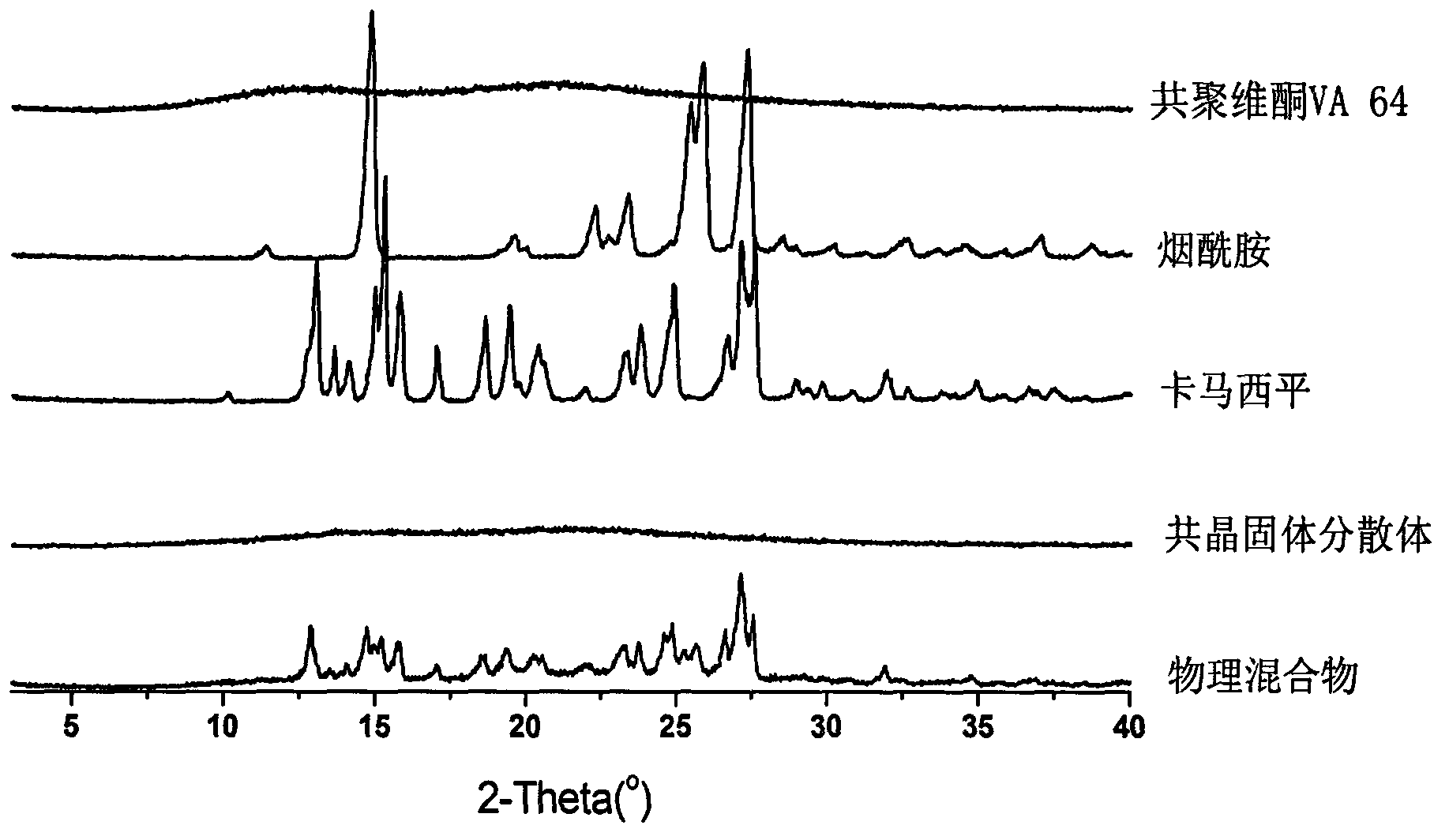
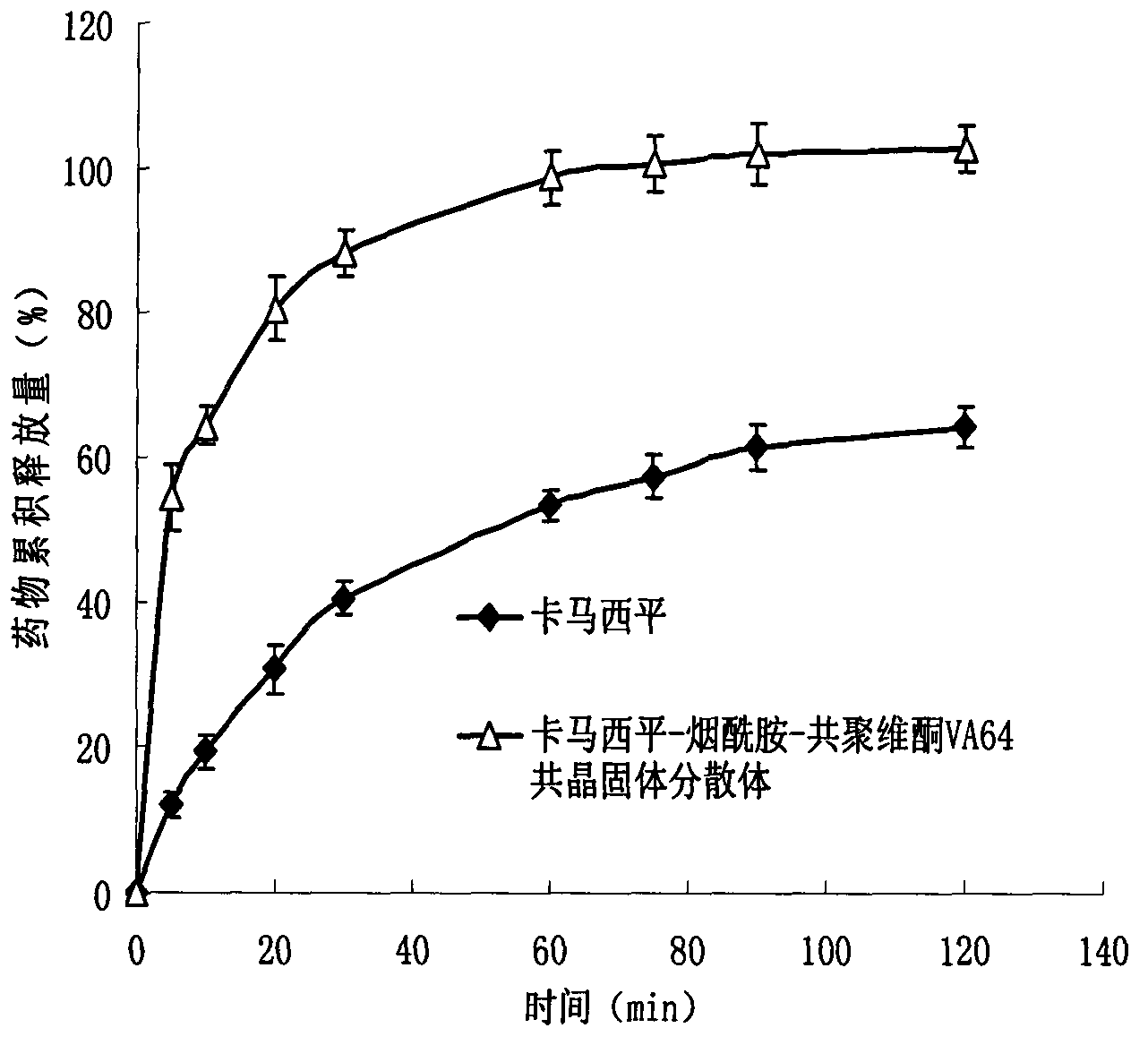
Carbamazepine cocrystal solid dispersion and preparation method thereof
A carbamazepine co-crystal and solid dispersion technology, which is applied in the field of medicine, can solve the problems of slow dissolution rate, poor absorption effect, and low bioavailability, and achieve fast dissolution rate, high dissolution rate, and reduced dosage. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 Carbamazepine eutectic solid dispersion
[0038] Prepared from the following components:
[0039] Carbamazepine (poorly soluble drug, Wuhan Yuancheng Technology Development Co., Ltd.) 2.72g
[0040] Niacinamide (eutectic ligand, Shanghai Jingchun Reagent Co., Ltd.) 1.29g
[0041] Copovidone VA64 (polymer carrier material, German BASF company) 4.01g
[0042] The preparation method of the carbamazepine eutectic solid dispersion of embodiment 1 is eutectic-melting method, comprises the following steps:
[0043] (1) Preparation of eutectic
[0044] Dissolve 0.0115mol (1.29g) of nicotinamide in 16ml of absolute ethanol, add 0.0115mol (2.72g) of carbamazepine, heat to 65°C until carbamazepine is completely dissolved, then cool down to 55°C to facilitate carbamazepine The carbamazepine-nicotinamide co-crystal is precipitated, and the temperature is further lowered to 20°C to promote the further precipitation of the crystal. The Buchner funnel is used to filter...
Embodiment 2
[0048] Embodiment 2 Carbamazepine eutectic solid dispersion
[0049] Prepared from the following components:
[0050] Carbamazepine (poorly soluble drug, Wuhan Yuancheng Technology Development Co., Ltd.) 2.72g
[0051] Niacinamide (co-crystal ligand, Shanghai Jingchun Reagent Co., Ltd.) 1.29g
[0052] Copovidone VA64 (polymer carrier material, German BASF company) 4.01g
[0053] The preparation method of the carbamazepine eutectic solid dispersion of embodiment 2 is eutectic-hot-melt extrusion method, comprises the following steps:
[0054] (1) Preparation of eutectic
[0055] Dissolve 0.0115mol (1.29g) of nicotinamide in 16ml of absolute ethanol, add 0.0115mol (2.72g) of carbamazepine, heat to 65°C until carbamazepine is completely dissolved, then cool down to 55°C to facilitate carbamazepine For the precipitation of the mazepine-nicotinamide co-crystal, the temperature is further lowered to 20°C to promote the further precipitation of the crystal, which is separated by s...
Embodiment 7
[0059] Embodiment 7 Carbamazepine eutectic solid dispersion
[0060] Prepared from the following components:
[0061] Carbamazepine (poorly soluble drug, Wuhan Yuancheng Technology Development Co., Ltd.) 2.72g
[0062] Niacinamide (co-crystal ligand, Shanghai Jingchun Reagent Co., Ltd.) 1.29g
[0063] Copovidone VA64 (polymer carrier material, German BASF company) 6.02g
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap