Method for preparing beta-amino carbonyl compound through synergetic catalysis of titanocene dichloride and aminophenol ligand
A technology of titanocene dichloride and aminocarbonyl, which is applied in the field of synergistic catalytic preparation of titanocene dichloride and aminophenol ligands to prepare β-aminocarbonyl compounds, achieving the effects of short reaction time, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
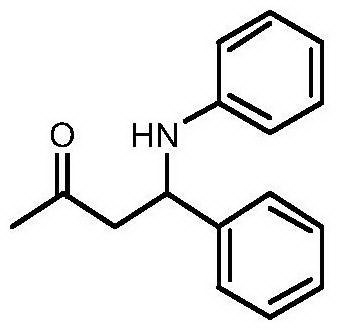
[0012] Taking the preparation of 4-phenyl-4-phenylamino-2-butanone with the following structural formula as an example, the raw materials used and the preparation method thereof are as follows:
[0013]
[0014] Put 102 μL (1.0 mmol) of benzaldehyde, 100 μL (1.1 mmol) of aniline, 2 mL (27 mmol) of acetone, 12.5 mg (0.05 mmol) of titanocene dichloride, and 10.9 mg (0.1 mmol) of p-aminophenol in a reaction flask, 25 After reacting at ℃ for 2 hours, the reaction was stopped and separated by column chromatography to obtain the white solid product 4-phenyl-4-phenylamino-2-butanone with a yield of 88%. The resulting product was characterized by a Bruker Avance superconducting Fourier digital NMR spectrometer, and the characterization data were: 1 H NMR (400MHz, CDCl 3 )δppm: 7.21-7.37 (m, 5H), 7.09 (t, J = 7.7Hz, 2H), 6.68 (t, J = 7.3Hz, 1H), 6.56 (d, J = 8.2Hz, 2H), 4.84 ( t, J=6.4Hz, 1H), 2.94(d, J=6.4Hz, 2H), 2.09(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ: 207.34, 146.71, 142.45...
Embodiment 2
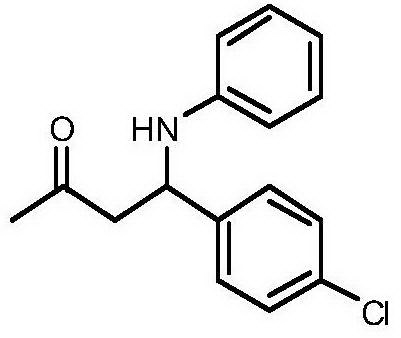
[0016] Taking the preparation of 4-(4-chlorophenyl)-4-phenylamino-2-butanone as an example with the following structural formula, the raw materials used and the preparation method thereof are as follows:
[0017]
[0018] In Example 1, the benzaldehyde used was replaced with equimolar p-chlorobenzaldehyde, and the other steps were the same as in Example 1 to prepare a yellow solid 4-(4-chlorophenyl)-4-phenylamino-2-butanol Ketone, its productive rate is 85%, and characteristic data is: 1 H NMR (400MHz, CDCl 3 )δppm: 7.22(m, 4H), 7.04(t, J=7.7Hz, 2H), 6.62(t, J=7.2Hz, 1H), 6.45(d, J=8.0Hz, 2H), 4.75(t, J=6.3Hz, 1H), 4.38(s, 1H), 2.85(d, J=6.3Hz, 2H), 2.05(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ: 206.69, 146.50, 141.11, 133.02, 129.19, 128.97, 127.73, 118.13, 113.79, 53.75, 51.02, 30.78.
Embodiment 3
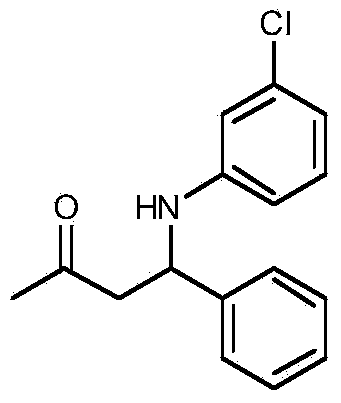
[0020] Taking the preparation of 4-phenyl-4-(3-chloroanilino)-2-butanone as an example, the raw materials used and the preparation method thereof are as follows:
[0021]
[0022] In Example 1, the aniline used was replaced with equimolar m-chloroaniline, and other steps were the same as in Example 1 to prepare yellow solid 4-phenyl-4-(3-chloroanilino)-2-butanone, Its yield is 94%, and the characteristic data are: 1 H NMR (400MHz, CDCl 3 )δppm: 7.32(m, 4H), 7.24(d, J=1.7Hz, 1H), 6.98(t, J=8.0Hz, 1H), 6.61(d, J=7.9Hz, 1H), 6.52(s, 1H), 6.40(d, J=8.2Hz, 1H), 4.80(s, 1H), 4.59(s, 1H), 2.91(d, J=6.3Hz, 2H), 2.08(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ: 206.96, 147.98, 141.86, 134.82, 130.12, 128.90, 127.55, 126.17, 117.72, 113.49, 111.87, 54.17, 50.89, 30.82.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com