Flavonoid derivative and application thereof
A technology of flavonoids and derivatives, applied to flavonoid derivatives and their application fields, can solve problems such as QT interval prolongation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
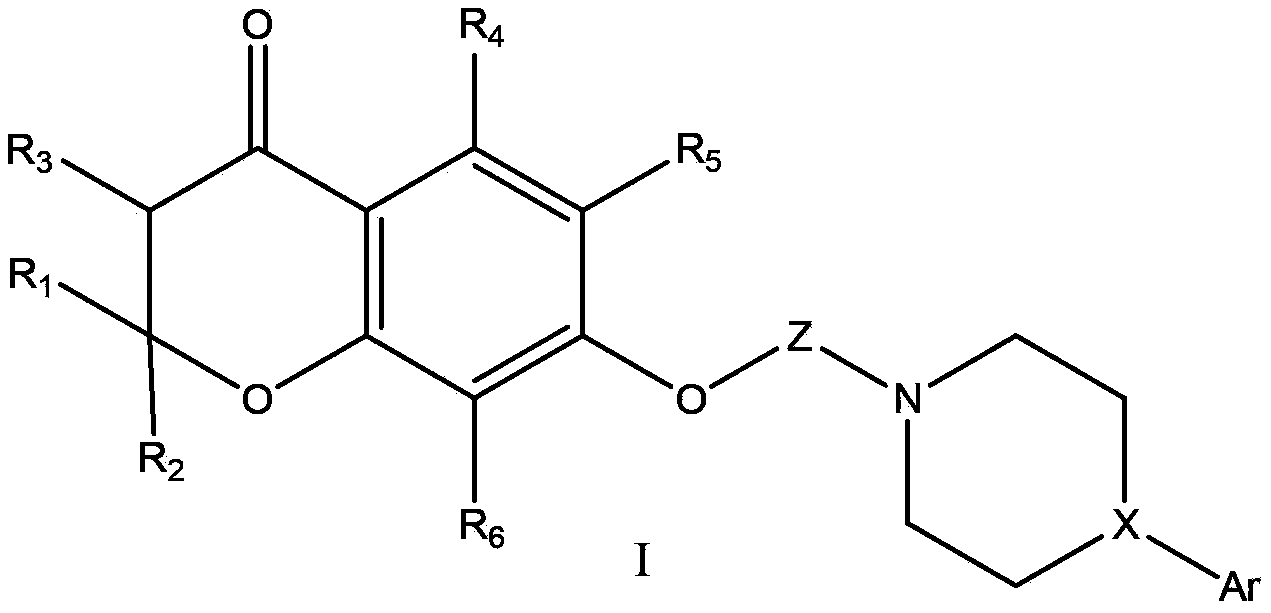
[0077] Example 1, 7-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butoxy)spiro[chroman-2-1'-cyclopentane]-4-one (1)
[0078] Reaction 1
[0079]
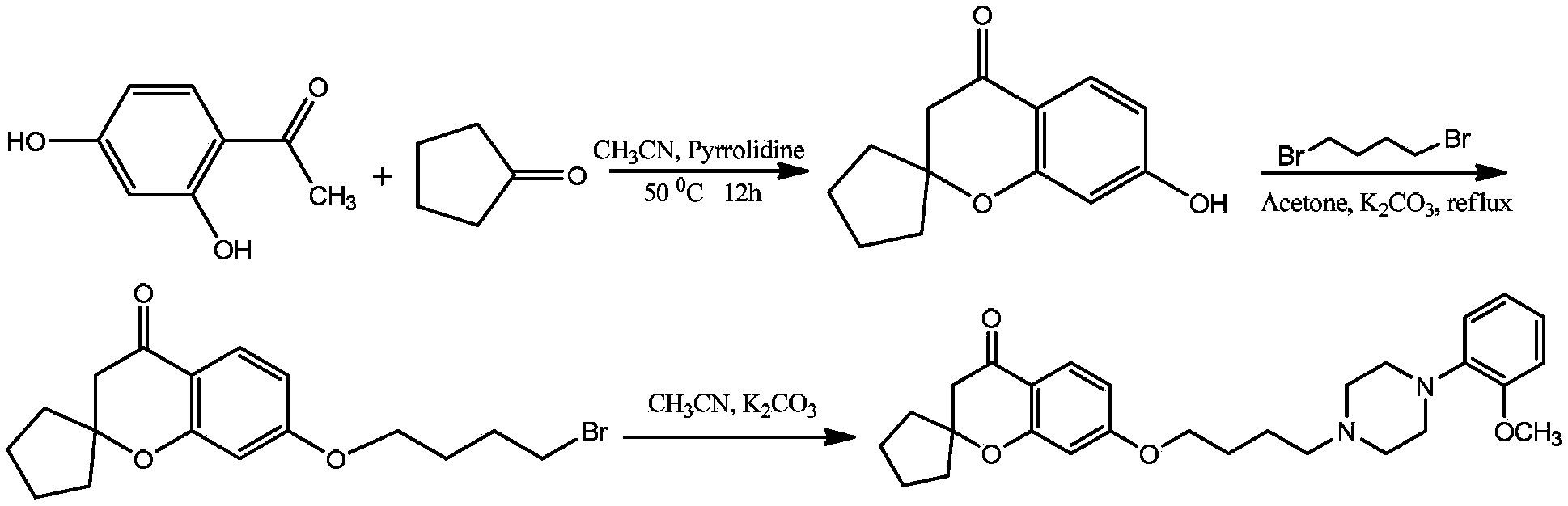
[0080] 1) Take 7.6g of 2,4-dihydroxyacetophenone, 8.4g of cyclopentanone, and 7.1g of tetrahydropyrrole, add 50ml of anhydrous acetonitrile, and react at 50°C for 12h. TLC detection, the reaction is completed, cooled to room temperature, the solution is slowly poured into 2M hydrochloric acid ice-water mixture, a solid precipitates, stirred for 30 minutes, filtered to obtain a yellow solid, recrystallized with 95% ethanol to obtain a white solid, and dried to obtain the product 8.4g, melting point 186‐188°C, yield 77.1%.
[0081] 2) Take 4.2g of the first step product, 6g of anhydrous potassium carbonate, 50ml of acetone, 8.2g of 1,4‐dibromobutane, heat and reflux for 6 hours, cool to room temperature, filter, evaporate the solvent to dryness, and use eluent Petroleum ether: ethyl acetate 4:1 was passed through the column to obtain 5.6 ...
Embodiment 2
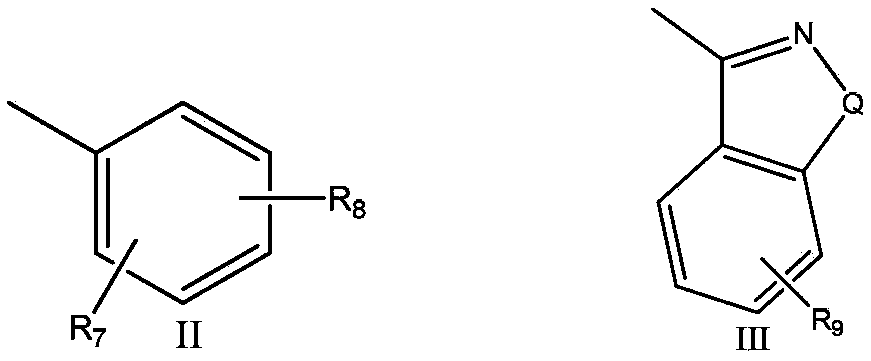
[0082] Example 2, 7-(4-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)butoxy)helix [chroman-2-1'- Cyclopentane]‐4‐one (2)
[0083] Replace 2-methoxyphenylpiperazine hydrochloride with 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride, prepared according to the method of Example 1 The target compound 2 was obtained: 7‐(4‐(4‐(6‐fluorobenzo[d]isoxazol‐3‐yl)piperidin‐1‐yl)butoxy)helix [chroman‐2‐1' ‐cyclopentane]‐4‐one.
[0084] Melting point: 103-105°C. 1 H NMR (CDCl 3 )δ1.63‐1.90(m,10H),2.07‐2.19(m,8H),2.48(t,2H,J=8Hz),2.78(s,2H),3.08‐3.12(m,4H),4.04( t, 2H, J=8Hz), 6.38(d, 1H, J=4Hz), 6.54-6.56(m, 1H), 7.04-7.09(m, 1H), 7.24-7.26(m, 1H), 7.69-7.73 (m,1H),7.80(d,1H,J=8Hz).MS (ESI) m / z493.3([M+H] + )
Embodiment 3
[0085] Example 3, 7-(3-(4-(2-methoxyphenyl)piperazin-1-yl)propoxy)spiro[chroman-2-1'-cyclopentane-4-one ( 3)
[0086] With 1,3-dibromopropane instead of 1,4-dibromobutane, target compound 3 was prepared according to the method of Example 1: 7-(3-(4-(2-methoxyphenyl)piperazine- 1-yl)propoxy)helix[chroman-2-1'-cyclopentane-4-one.
[0087] Melting point: 128-130°C. 1 H NMR (CDCl 3 )δ1.65‐1.74 (m, 4H), 1.87‐1.91 (m, 2H), 2.03‐2.12 (m, 4H), 2.61 (t, 2H, J=8Hz), 2.71 (s, br, 4H), 2.79(s,2H),3.13(s,br,4H),3.89(s,3H),4.09(t,2H,J=8Hz),6.41(d,1H,J=4Hz),6.55‐6.58(m ,1H),6.88‐7.04(m,4H),7.81(d,1H,J=8Hz).MS (ESI) m / z451.2([M+H] + )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com