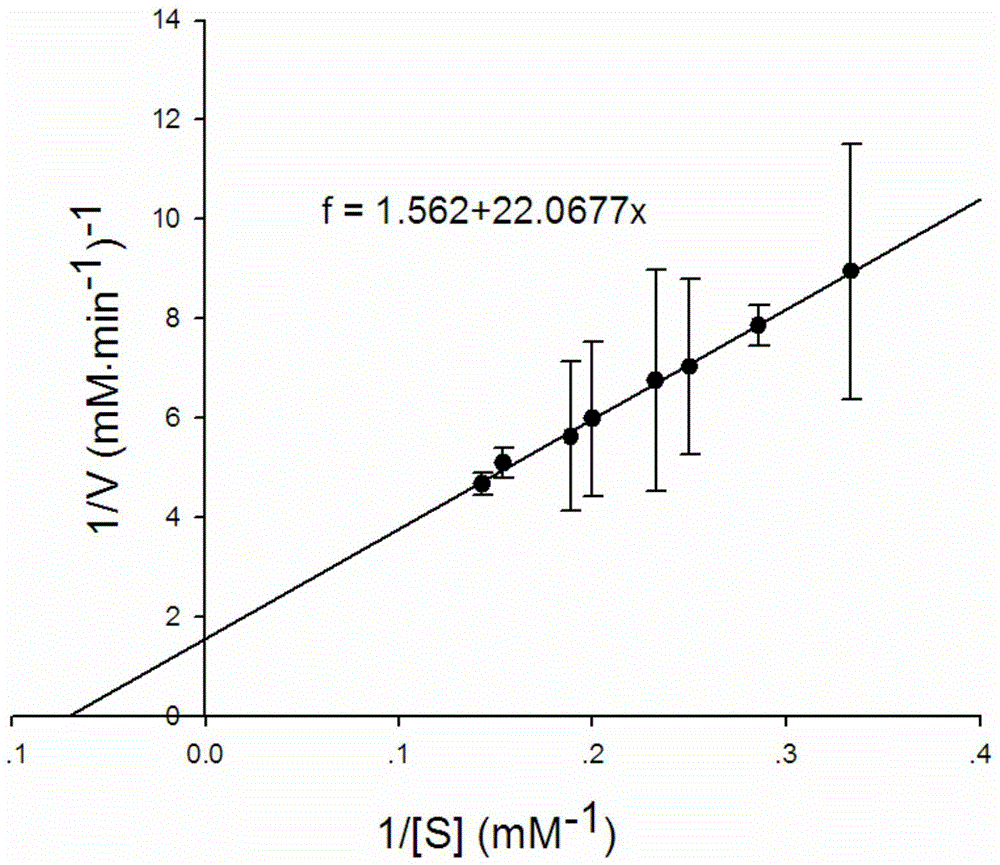
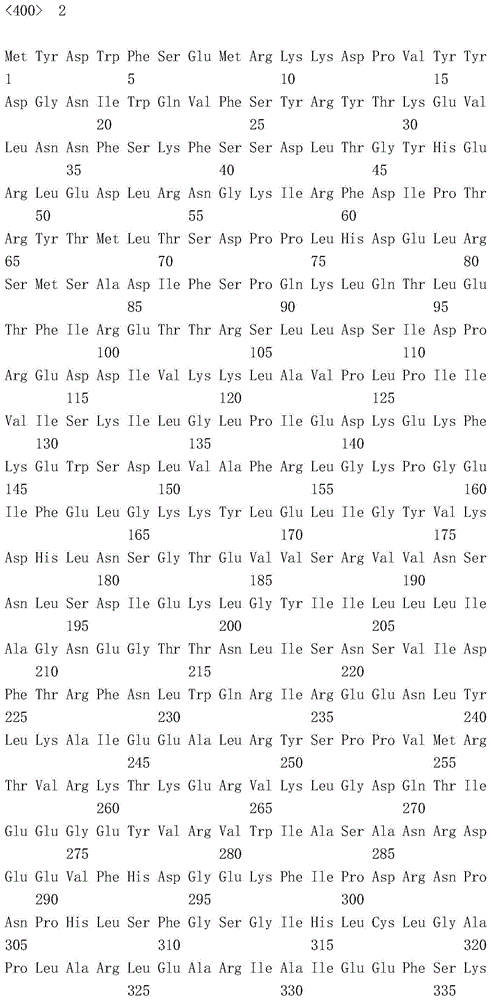Cyp119-t213g enzyme and purification method thereof
A CYP119-T213G, purification method technology, applied in the field of bioengineering, can solve problems such as the reduction of catalytic efficiency, and achieve the effects of improving epoxidation reaction, high corresponding selectivity, and wide application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 CYP119-T213G enzyme expression vector construction
[0018] Mutation of CYP119 enzyme: The primer sequences of the mutation site designed according to the pET30a-CYP119-T213G vector are: SEQ ID No.3 and SEQ ID No.4. The QuickChange Lighting Site-directed MutagenesisKit site-directed mutagenesis kit was used for site-directed mutagenesis, and E. coli DH5α was transformed. Select positive clones for sequencing to confirm whether the mutation is completed and the accuracy of the mutated site.
[0019] SEQ ID No.3: Upstream primer for amplifying CYP119-T213G enzyme
[0020] 5'-TTCTCATAGCGGGTAATGAG ACAACTAACTTAATATCAAA-3', the bold underlined site is the mutation site.
[0021] SEQ ID No.4: Downstream primer for amplifying CYP119-T213G enzyme
[0022] 5'-TTTGATATTAAGTTAGTTGT CTCATTACCCGCTATGAGAA-3', the bold underlined site is the mutation site.
Embodiment 2
[0023] A large amount of expression of embodiment 2 CYP119-T213G enzyme
[0024] The pET30a-CYP119-T213G plasmid was transformed into BL21(DE3)plysS Escherichia coli competent cells. Select positive single colonies, inoculate them in 5 mL double-antibody LB liquid medium, and culture overnight at 37°C with shaking. Take 2 mL of the overnight cultured bacterial liquid and put it in 1 L of double-antibody TB culture medium. Add 250 μl / L Trace Element, culture at 37°C with shaking until OD0.6. Add 0.4mM IPTG to a final concentration of 0.4mM, induce at 32°C for 45h.
Embodiment 3
[0025] The purification of embodiment 3CYP119-T213G enzyme
[0026] Take the bacteria liquid fermented in Example 2, centrifuge at 12000 rpm, 4°C for 10 min, discard the supernatant, add 20 mL pH7.4, 50 mMPBS solution to fully suspend the bacteria. Place the sample on ice water, and use an ultrasonic cell breaker with 50% power, 3s-3s, 20 minutes to break it twice. After cell destruction, heat in a water bath at 55°C for 15 minutes, centrifuge at 12,000 rpm at 4°C for 40 minutes, and take the supernatant as the crude enzyme solution.
[0027] Then the crude enzyme solution is purified, and the equilibrium solution I is eluted for 5 column volumes; the crude enzyme solution filtered by a 0.22 μm filter membrane is put on the column; the equilibrium solution I is washed for 5 column volumes; the equilibrium solution II is washed for 5 column volumes; Wash 6 column volumes with eluent I; wash 5 column volumes with eluent II, and collect the eluate.
[0028] The collected eluate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com