Silver-coated copper alloy powder and method for manufacturing same
A technology of copper alloy and powder, which is applied in the field of silver-coated copper alloy powder and its production, can solve the problems of high volume resistivity, copper powder storage stability (insufficient reliability, low conductivity, etc.), and achieve low volume resistivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
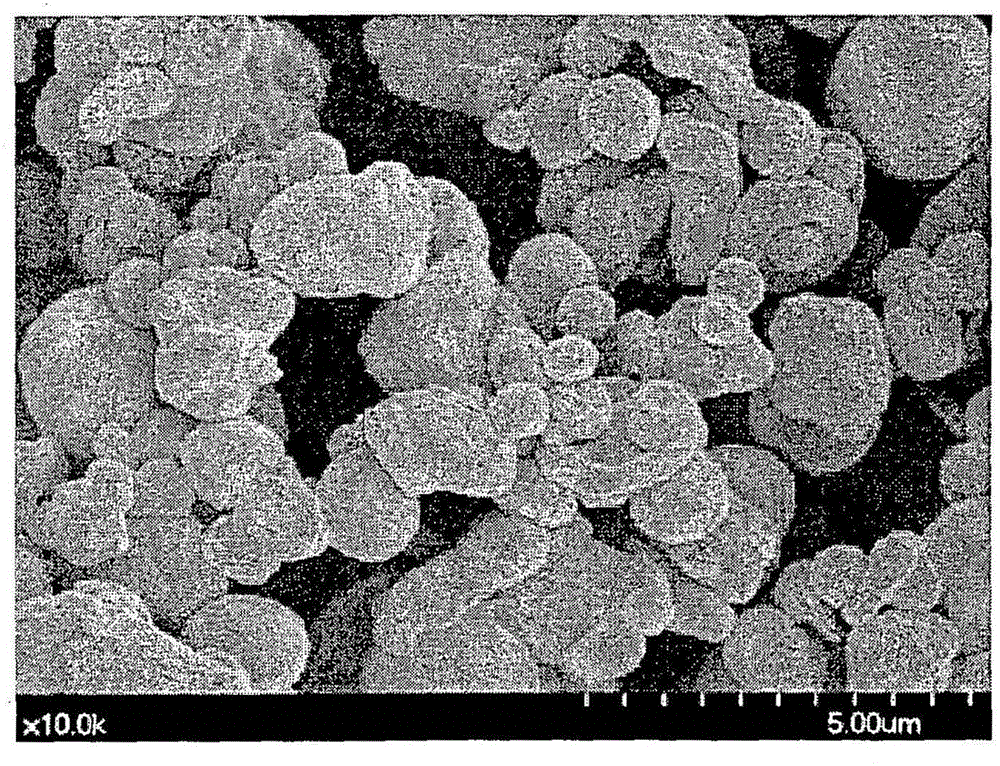
[0033] The molten metal obtained by heating 7.2 kg of copper and 0.8 kg of nickel was rapidly cooled and solidified by spraying high-pressure water thereon while the molten metal dripped from the lower part of the refractory tank. The alloy powder thus obtained was filtered, washed with water, dried and crushed to obtain copper alloy powder (copper-nickel alloy powder).
[0034] Then, 61.9 g of dihydrate EDTA-2Na and 61.9 g of ammonium carbonate were dissolved in 720 g of pure water to prepare a solution (solution 1), and to 2097 g of pure water was dissolved 263.2 g of dihydrate EDTA-2Na and 526.4 A solution obtained by dissolving 87.7 g of silver nitrate in 271 g of pure water was added to a solution obtained by g of ammonium carbonate to prepare a solution (solution 2).
[0035] Then, under a nitrogen atmosphere, 130 g of the obtained copper-nickel alloy powder was added to Solution 1, and the temperature of the solution was raised to 35° C. while stirring the solution. Af...
Embodiment 2
[0062] Copper-nickel alloy powder coated with silver (copper alloy powder coated with silver) was obtained by the same method as in Example 1 using the same copper alloy powder (copper-nickel alloy powder) as in Example 1, except that , a solution prepared by dissolving 61.9 g of EDTA-2Na dihydrate and 61.9 g of ammonium carbonate in 720 g of pure water was used as solution 1, and by dissolving 307.1 g of EDTA-2Na dihydrate in 1223 g of pure water A solution prepared by dissolving 51.2 g of silver nitrate in 222 g of pure water was added to a solution prepared with 153.5 g of ammonium carbonate and used as solution 2.
[0063]For the silver-coated copper alloy powder thus obtained, the composition of the powder, the amount of silver coated therein, the average particle size thereof, and the electrical resistance of the pressed powder thereof were obtained by the same method as in Example 1, and by the same method as in Example 1 The storage stability (reliability) of the powde...
Embodiment 3
[0067] Copper-nickel alloy powder coated with silver (copper alloy powder coated with silver) was obtained by the same method as in Example 1 using the same copper alloy powder (copper-nickel alloy powder) as in Example 1, except that , a solution prepared by dissolving 19 g of EDTA-2Na dihydrate and 19 g of ammonium carbonate in 222 g of pure water was used as solution 1, and by dissolving 252 g of EDTA-2Na dihydrate and 126 g of ammonium carbonate in 1004 g of pure water To the prepared solution was added a solution prepared by dissolving 42 g of silver nitrate in 100 g of pure water was used as solution 2.
[0068] For the silver-coated copper alloy powder thus obtained, the composition of the powder, the amount of silver coated therein, the average particle size thereof, and the electrical resistance of the pressed powder thereof were obtained by the same method as in Example 1, and by the same method as in Example 1 The storage stability (reliability) of the powder was ev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com