A kind of oxiracetam for injection and preparation method thereof
A technology of water for injection and control bottles, which is applied in the direction of freeze-drying transportation, neurological diseases, powder transportation, etc. It can solve the problems of long freeze-drying cycle, unfavorable drying, slow drying, etc., and achieve low moisture content, low impurity level and high quality. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Prepare oxiracetam solution at a concentration of 50%, add ethanol of 0%, 1.0%, 1.2%, 1.4%, 1.6%, 1.8%, 2.0% and 2.2% (V / V) of the total volume respectively, observe the oxiracetam Dissolution of Piracetam. The results are shown in Table 1:
[0052] Ethanol concentration (V / V)
[0053] From the above experimental results, it can be seen that when the amount of ethanol is greater than 2.0%, oxiracetam cannot be completely dissolved, and its solubility decreases. Therefore, during the preparation of this product, the amount of ethanol should not exceed 2.0%.
Embodiment 2
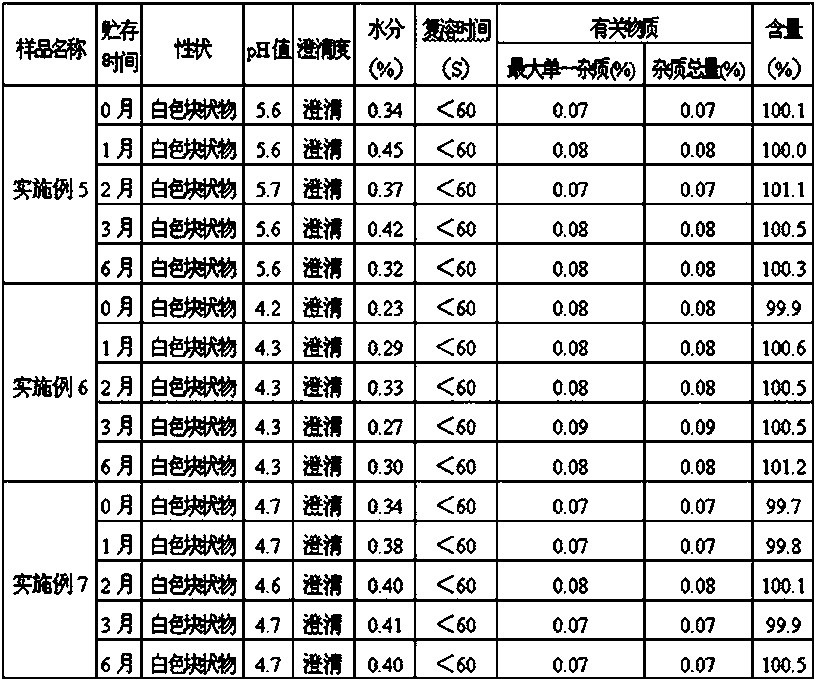
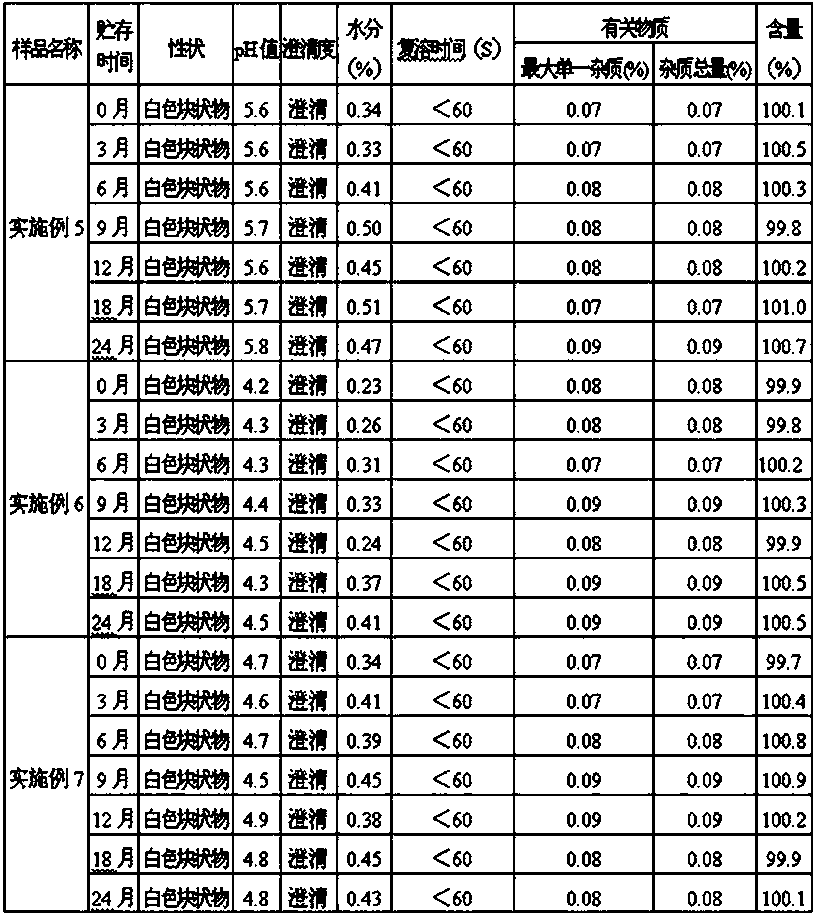
[0055] On the basis of Example 1, store the completely dissolved drug solution at 60°C for 24 hours, and take a sample at the specified time to detect the amount of impurity (oxiracetam acid). The results are shown in Table 2:
[0056] Table 2 The impurity levels of oxiracetam in solutions containing different amounts of ethanol
[0057]
[0058] From the above experimental results, it can be seen that the amount of oxiracetam acid increases with time, and the amount of increase is related to the amount of ethanol in the solution. The more the amount of ethanol, the less the amount of increase. When the amount of ethanol is greater than or equal to 1.4%, the amount of increase is the least, and oxiracetam is the most stable.
Embodiment 3
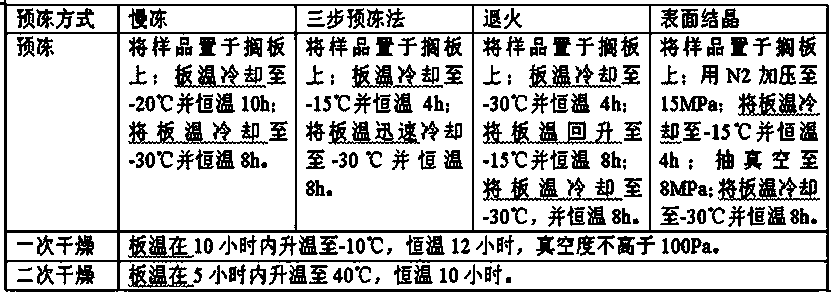
[0060] On the basis of Example 1, carry out terminal filtration and sterilization of the completely dissolved medicinal liquid through a 0.22 μm microporous membrane to obtain a filtrate, fill the filtrate into a control bottle at 5.0 ml / bottle, stopper, and use ordinary pre-freezing Freeze-drying is carried out after the method, and the freeze-drying program is shown in Table 3:
[0061] pre-freeze
[0062] Statistically obtain the ratio of frozen concentrated layer samples in each batch of samples, the results are shown in Table 4:
[0063] Ethanol concentration (V / V)
[0064] From the above experimental results, it can be seen that all the samples without ethanol have freeze-concentrated layers. For samples containing ethanol, as the amount of ethanol increases, the proportion of samples with frozen concentrate layer decreases. When the amount of ethanol is greater than or equal to 1.4%, the proportion of samples with frozen concentrated layer is small....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com