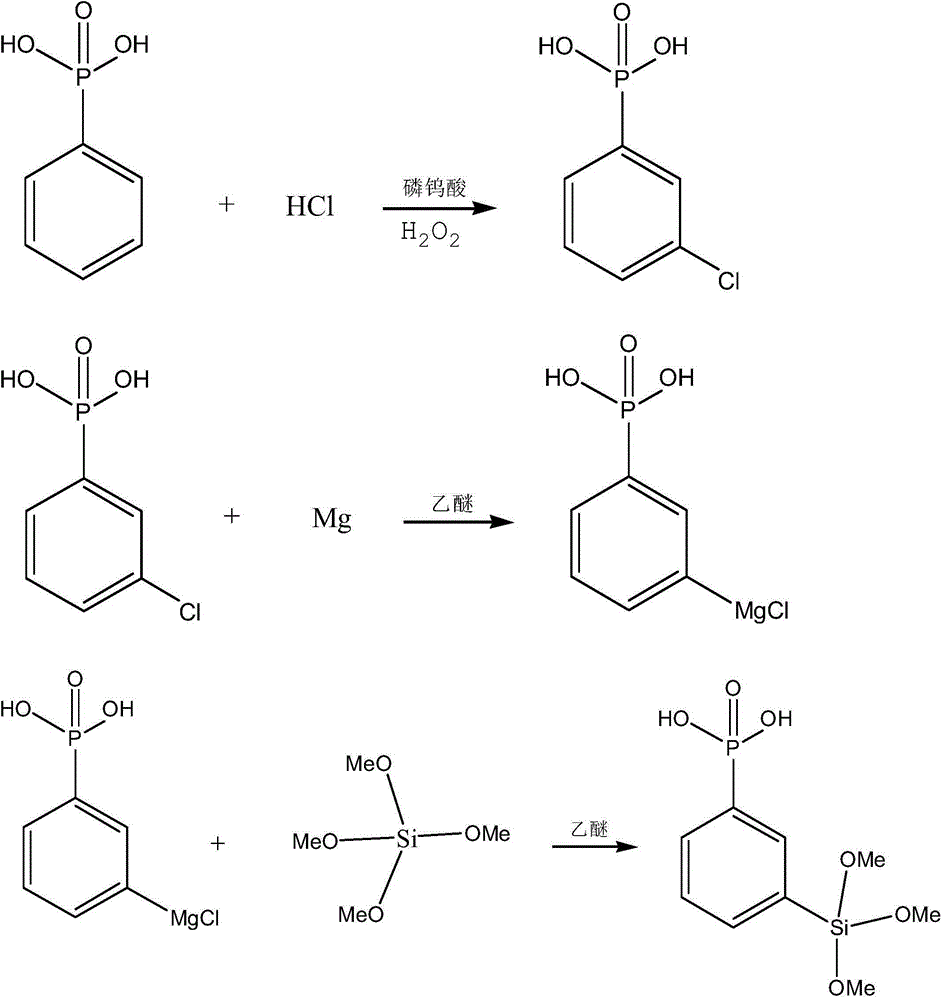
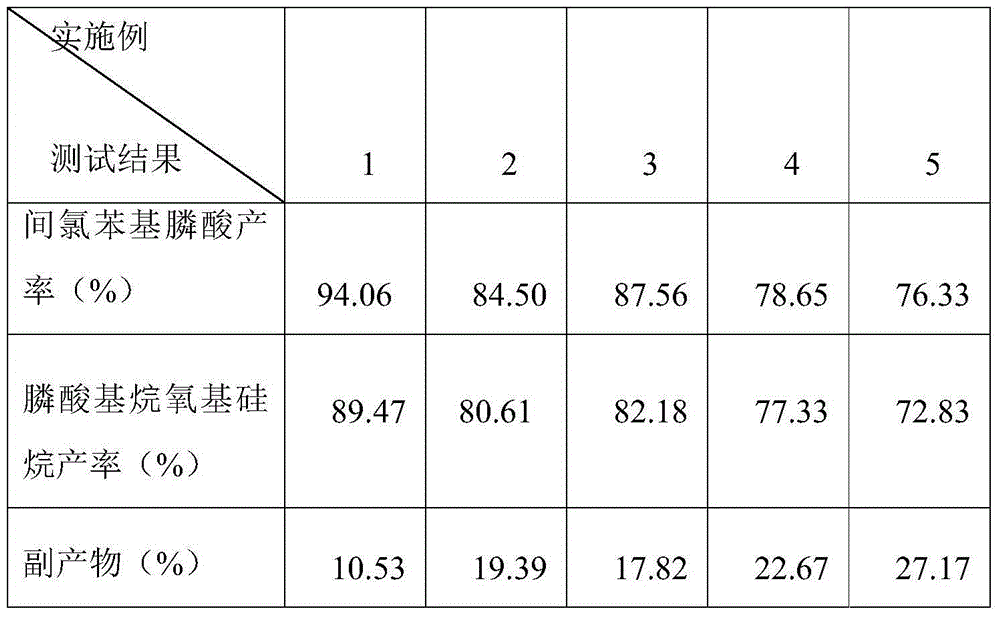
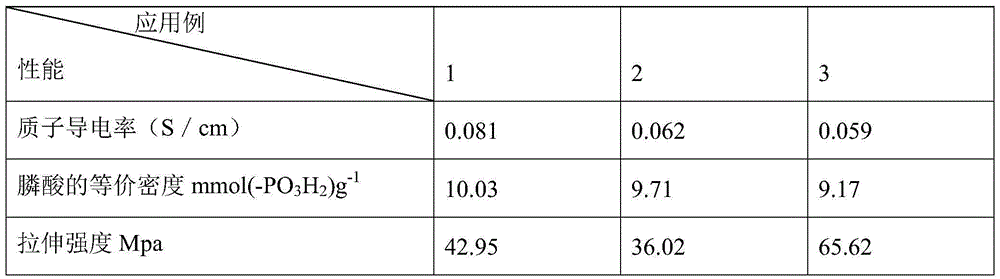
Grignard reaction-based method for preparing phenylphosphonic acid trimethoxy silane
A technology of phenylphosphonic acid trimethoxysilane and tetramethoxysilane, which is applied in the field of preparation of phenylphosphonic acid trimethoxysilane, can solve the problem that the keying success rate cannot be guaranteed, the proton conductivity is not high, the content of phosphonic acid Stability limitations and other issues, to achieve the effect of less side reactions, stable phosphonic acid content, and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation method of the phenylphosphonic acid trimethoxysilane based on Grignard reaction, it comprises the steps:
[0023] 1) Under the condition of anhydrous and nitrogen protection, add 3g of dry magnesium powder and 2g into the flask, and add 10mL of anhydrous ether and 5mL of m-chlorophenylphosphonic acid, heat the solution for a few minutes and make the solution slightly boil, and wait for the reaction After the reflux of anhydrous ether in the medium was evaporated, add 15mL m-chlorophenylphosphonic acid and 15mL anhydrous ether dropwise, continue to react for 1h after the dropwise addition, then slowly heat and recover anhydrous ether, until the reaction temperature reaches 80°C, Stop the reaction to obtain the Grignard reagent of m-chlorophenylphosphonic acid; wherein the average temperature rise rate of slow heating is 10° C. / min;
[0024] 2) Under the conditions of anhydrous and nitrogen protection, the prepared Grignard reagent of m-chlorophenylphospho...
Embodiment 2
[0026] The preparation method of the phenylphosphonic acid trimethoxysilane based on Grignard reaction, it comprises the steps:
[0027] 1) Under the condition of anhydrous and nitrogen protection, add 3g of dry magnesium powder and several grains of iodine into the flask, and add 10mL of anhydrous ether and 5mL of m-chlorophenylphosphonic acid, heat the solution for a few minutes to slightly boil, After the reflux of anhydrous ether in the reaction is evaporated, add 15mL of m-chlorophenylphosphonic acid and 15mL of anhydrous ether dropwise; ℃, stop the reaction, and obtain the Grignard reagent of m-chlorophenylphosphonic acid; wherein the average temperature rise rate of slow heating is 7°C / min;
[0028] 2) Under the conditions of anhydrous and nitrogen protection, cool and stir the Grignard reagent of m-chlorophenylphosphonic acid on an ice-water bath, and at the same time, add 9.5mL tetramethoxysilane and 10mL anhydrous ether dropwise with a dropping funnel Control the ra...
Embodiment 3
[0030] The preparation method of the phenylphosphonic acid trimethoxysilane based on Grignard reaction, it comprises the steps:
[0031] 1) Under the condition of anhydrous and nitrogen protection, add 3g of dry magnesium powder and several grains of iodine into the flask, and add 10mL of anhydrous ether and 5mL of m-chlorophenylphosphonic acid, heat the solution for a few minutes to slightly boil, After the reflux of anhydrous ether disappears during the reaction, add 15mL of m-chlorophenylphosphonic acid and 15mL of anhydrous ether dropwise; after the dropwise addition, continue the reaction for 1 hour, then heat slowly and recover anhydrous ether until the reaction temperature reaches 80°C , stop the reaction to obtain the Grignard reagent of m-chlorophenylphosphonic acid; wherein the average temperature rise rate of slow heating is 10° C. / min;
[0032]2) Under the conditions of anhydrous and nitrogen protection, cool and stir the Grignard reagent of m-chlorophenylphosphoni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com