Fischer-Tropsch synthesis iron-based catalyst, preparation method and application thereof
An iron-based catalyst, Fischer-Tropsch synthesis technology, applied in chemical instruments and methods, preparation of liquid hydrocarbon mixtures, catalysts for physical/chemical processes, etc., can solve problems such as limited industrial application, low natural reserves, high Fischer-Tropsch activity, Achieve the effect of reducing preparation cost, low deactivation rate, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
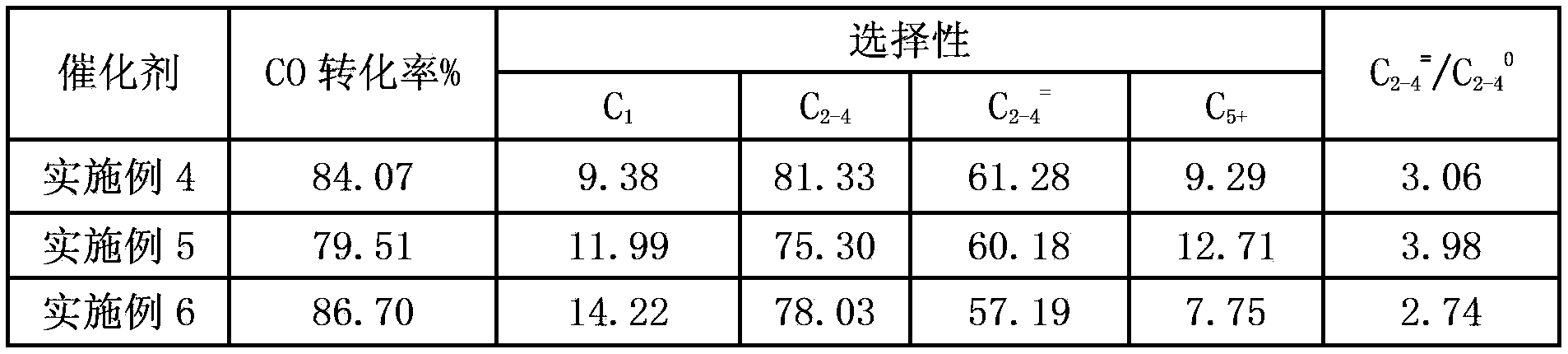
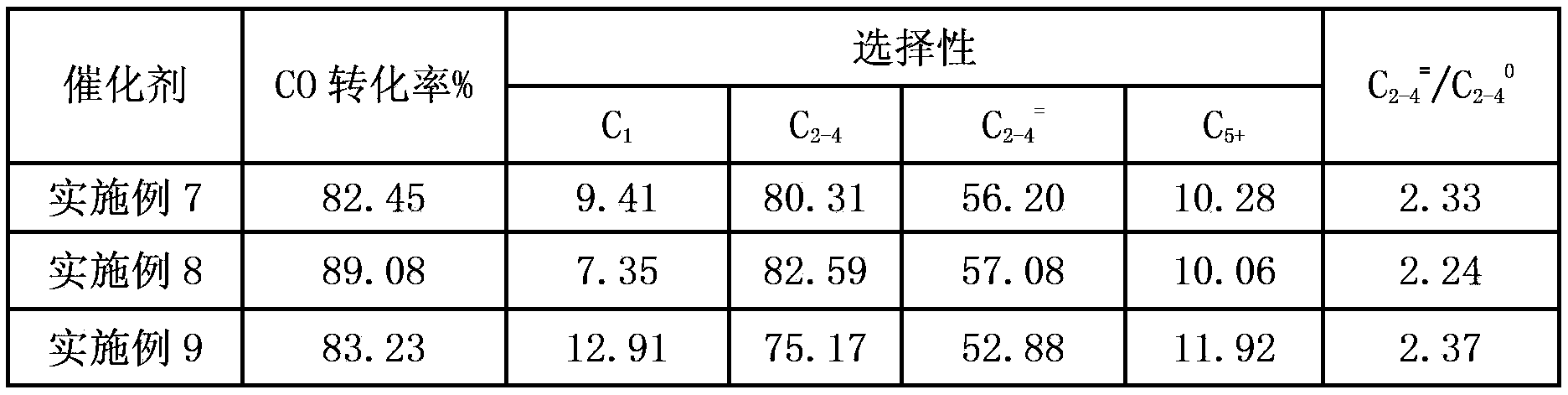
Examples
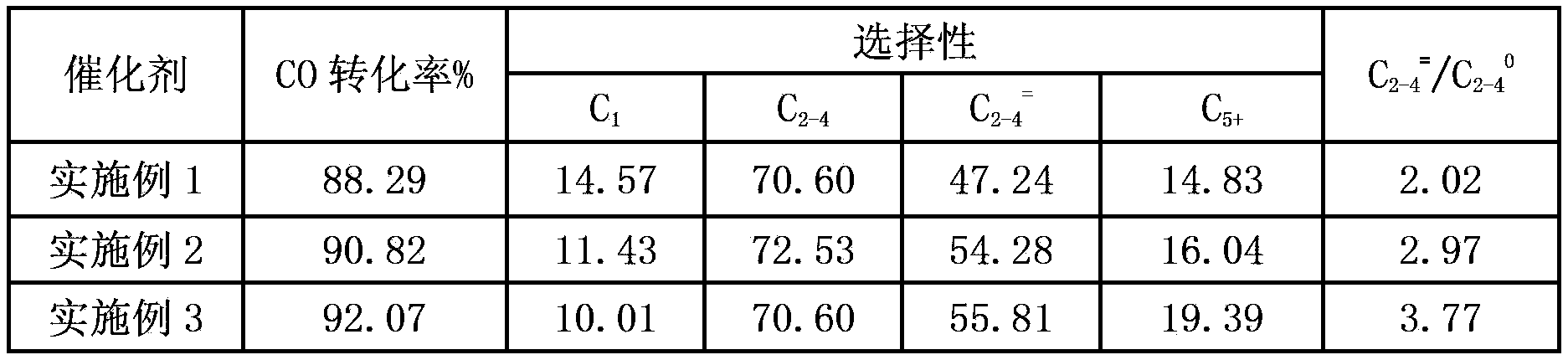
Embodiment 1
[0041] A preparation method for Fischer-Tropsch synthesis iron-based catalyst, comprising the steps of:
[0042] Weigh 16.1687 grams of Fe (NO 3 ) 3 9H 2 O, then weigh Cu (NO 3 ) 2 ·3H 2O, use deionized water to prepare a mixed salt solution with a total concentration of 0.1 mol / liter, place the mixed solution in a water bath at 50°C to fully dissolve it; prepare a certain amount of ammonia solution with a concentration of 1.0 mol / liter, Dissolve it fully in a water bath at 50°C; slowly co-currently titrate the above mixed solution and ammonia solution. During the titration process, the temperature is controlled at 35°C and the pH is 7.0. The precipitation titration process takes 1 hour. When the precipitation is terminated, The pH value of the system is 8.0; after the precipitation is completed, let it stand for 2 hours, filter with suction, wash the filter cake with deionized water until the pH value of the filtrate is 7.0, then filter with suction, and dry the filter c...
Embodiment 2
[0046] A preparation method for Fischer-Tropsch synthesis iron-based catalyst, comprising the steps of:
[0047] Weigh 16.1632 grams of Fe (NO 3 ) 3 9H 2 O, then weigh Cu(NO 3 ) 2 ·3H 2 O and Mn(NO 3 ) 2 4H 2 O, use deionized water to prepare a mixed salt solution with a total concentration of 0.5 mol / liter, place the mixed solution in a water bath at 50°C to fully dissolve it; prepare a certain amount of ammonia solution with a concentration of 1.0 mol / liter, Dissolve it fully in a water bath at 70°C; slowly co-current titrate the above mixed solution with ammonia solution, control the temperature at 50°C and pH 9.0 during the titration process, and take 1 hour for the precipitation titration process. The pH value of the system is 9.0; after the precipitation is completed, let it stand for 10 hours, filter with suction, wash the filter cake with deionized water until the pH value of the filtrate is 8.5, then filter with suction, and dry the filter cake at 90°C for 36 ...
Embodiment 3
[0051] A preparation method for Fischer-Tropsch synthesis iron-based catalyst, comprising the steps of:
[0052] Weigh 16.1629 grams of Fe (NO 3 ) 3 9H 2 O, then weigh Cu(NO 3 ) 2 ·3H 2 O and Mn(NO 3 ) 2 4H 2 O, use deionized water to prepare a mixed salt solution with a total concentration of 1.5 mol / liter, place the mixed solution in a water bath at 70°C to fully dissolve it; prepare a certain amount of ammonia solution with a concentration of 1.0 mol / liter, Dissolve it fully in a water bath at 70°C; slowly co-currently titrate the above mixed solution with ammonia solution. During the titration process, the temperature is controlled at 70°C, the pH is 11.0, and the precipitation titration process takes 1 hour. When the precipitation is terminated The pH value of the system is 11.5; after the precipitation is completed, let it stand for 15 hours, filter with suction, wash the filter cake with deionized water until the pH value of the filtrate is 9.0, then filter with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com