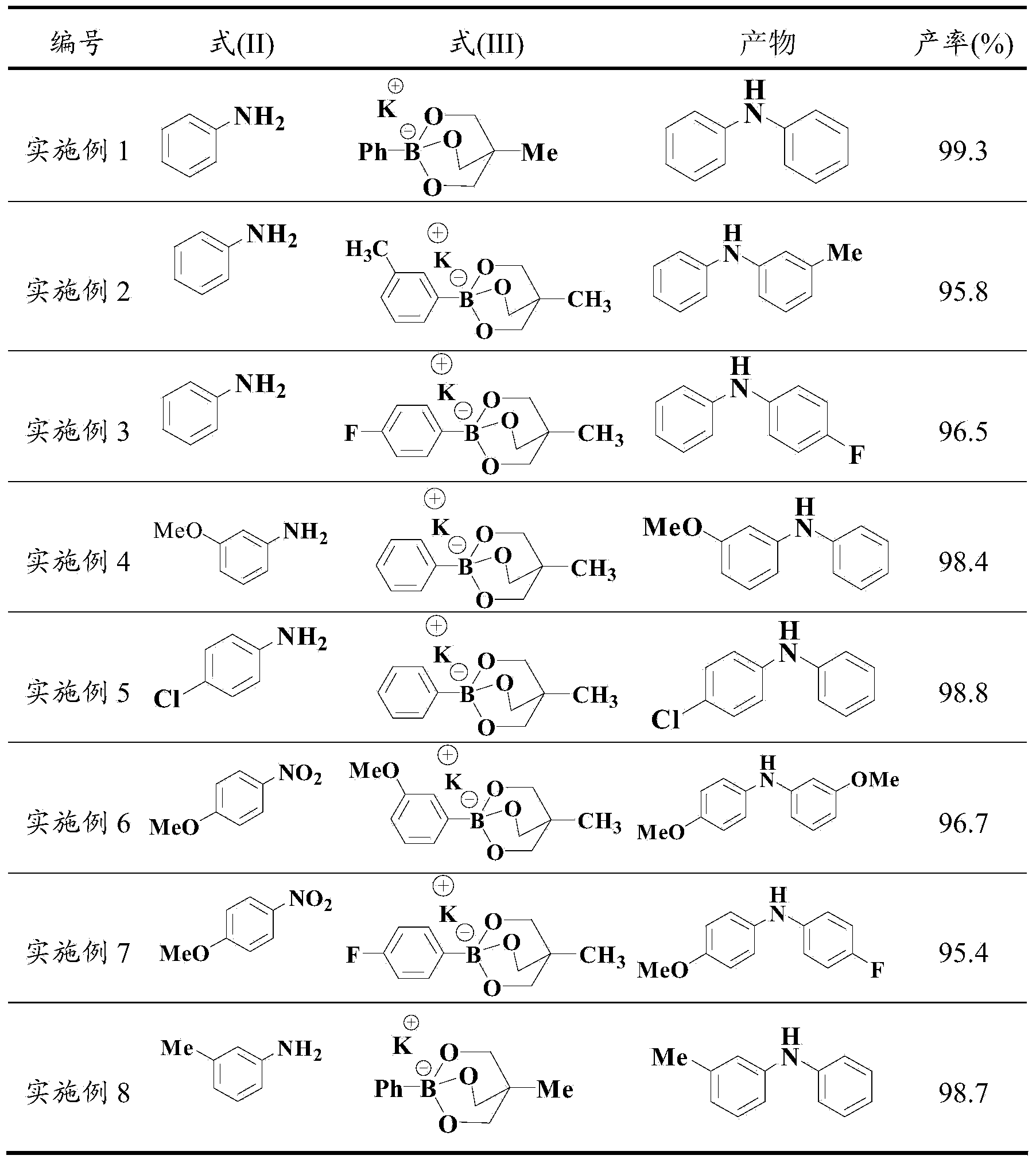Composite method for amine compound serving as medicine structuring unit
A synthetic method and compound technology, which is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of aminohydroxyl compounds, can solve the problems of long reaction steps, low yield, and less research, and achieve good scientific research value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
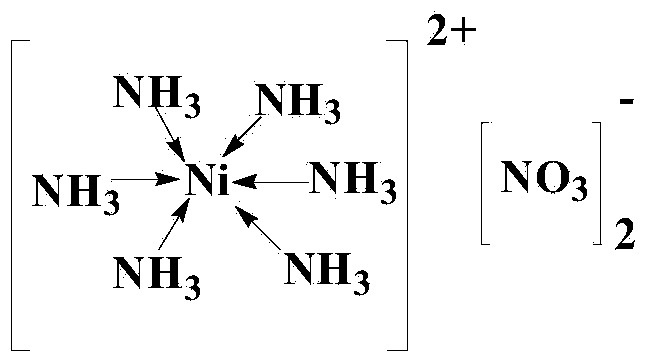
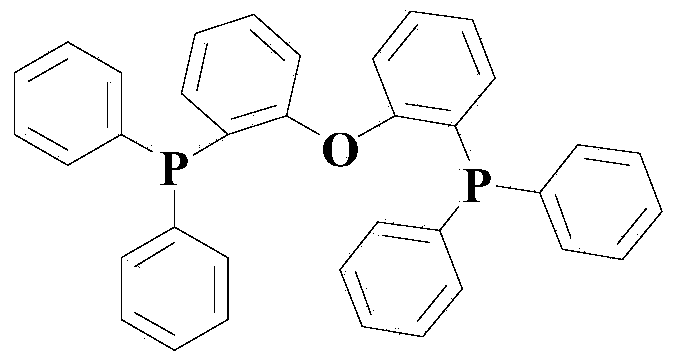
[0039] In the reaction vessel, add an appropriate amount but sufficient for the organic solvent DMF required for the reaction, then add the compounds of formula (II) and formula (III) corresponding to Example 1 in Table 1, and then add the nickel complex and the phosphine ligand; Wherein the molar ratio of formula (II) and formula (III) compound is 1:1, the molar ratio of formula (II) and nickel complex is 1:0.04, the molar ratio of nickel complex and phosphine ligand is 1:2; Nitrogen is purged thoroughly, and then nitrogen filling and replacement are continued for 2-3 times until the reaction vessel is completely filled with nitrogen, and heated at 70°C for 14 hours. After the reaction reaches the time, filter while it is hot, then use a rotary evaporator to remove the reaction solvent from the filtrate, and the resulting residue is separated by silica gel column chromatography, and the eluent is a mixed solvent of ethyl acetate and acetone with a volume ratio of 1:1. The elu...
Embodiment 2
[0041] In the reaction vessel, add an appropriate amount but sufficient for the organic solvent DMF required for the reaction, then add the compounds of formula (II) and formula (III) corresponding to Example 2 in Table 1, and then add the nickel complex and the phosphine ligand; Wherein the molar ratio of formula (II) and formula (III) compound is 1:2, the molar ratio of formula (II) and nickel complex is 1:0.07, the molar ratio of nickel complex and phosphine ligand is 1:3; Nitrogen is purged thoroughly, and then nitrogen filling and replacement are continued for 2-3 times until the reaction vessel is completely filled with nitrogen, and the reaction is heated at 90°C for 10 hours. After the reaction reaches the time, filter while it is hot, then use a rotary evaporator to remove the reaction solvent from the filtrate, and the resulting residue is separated by silica gel column chromatography, and the eluent is a mixed solvent of ethyl acetate and acetone with a volume ratio ...
Embodiment 3
[0043] In the reaction vessel, add an appropriate amount but sufficient for the organic solvent DMF required for the reaction, then add the compounds of formula (II) and formula (III) corresponding to Example 3 in Table 1, and then add the nickel complex and the phosphine ligand; Wherein the molar ratio of formula (II) and formula (III) compound is 1:3, the molar ratio of formula (II) and nickel complex is 1:0.1, the molar ratio of nickel complex and phosphine ligand is 1:2; Nitrogen was purged thoroughly, and then nitrogen filling and replacement were continued for 2-3 times until the reaction vessel was completely filled with nitrogen, and the reaction was heated at 110°C for 8 hours. After the reaction reaches the time, filter while it is hot, then use a rotary evaporator to remove the reaction solvent from the filtrate, and the resulting residue is separated by silica gel column chromatography, and the eluent is a mixed solvent of ethyl acetate and acetone with a volume rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com