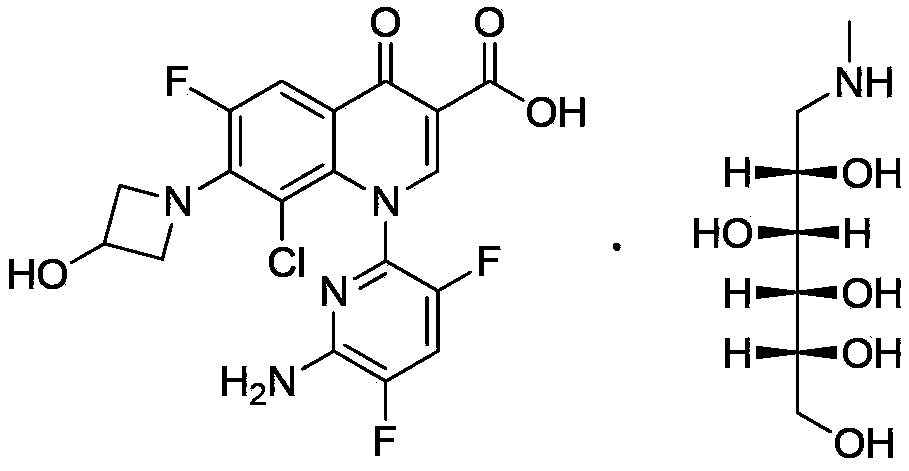
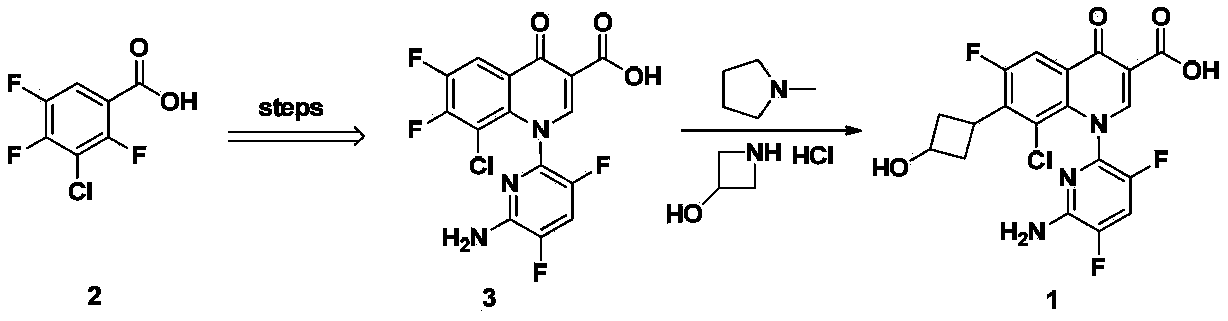
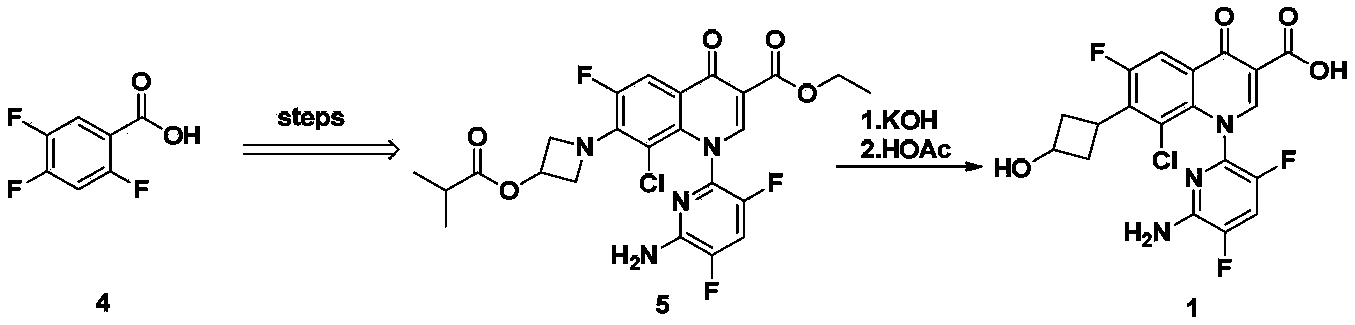
Delafloxacin purifying method
A purification method, a polar aprotic technology, applied in the direction of organic chemistry, etc., can solve the problems such as the inability to reach the medicinal standard, no delafloxacin purification method, etc., and achieve the effect of increasing the preparation cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 compound 1
[0028] According to the method described in Example 70 of patent WO9711068: add 2.00 g of 1-(6-amino-3,5-difluoropyridine- 2-yl)-8-chloro-6,7-difluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid, 1.00 g of 3-hydroxyazetidine hydrochloride and 2.00 g of N-methylpyrrolidine, the resulting mixture was stirred at 85° C. for 10 minutes, and the solvent and the like were distilled off under reduced pressure. Add 10mL of ethanol to the obtained residue, heat to reflux for 10 minutes and let cool, filter the precipitate, wash with ethanol and diisopropyl ether successively to obtain 2.1g of light yellow powder, HPLC purity 95.3%.
Embodiment 2
[0029] The refining of embodiment 2 compound 1
[0030] Add 13g of the crude product of Formula 1 prepared according to Example 1 to 39mL of N,N-dimethylformamide, heat to 100°C and stir to dissolve, slowly add distilled water in batches until it just appears turbid, a total of about 8.5mL, and slowly lower to room temperature , left to cool and crystallize for 8 hours, filtered, the filter cake was washed with ethanol, and spin-dried to obtain 10.9 g of light yellow powder, yield 84%, HPLC purity: 99.5%.
Embodiment 3
[0031] The refining of embodiment 3 compound 1
[0032] Add 13g of the crude intermediate of formula I prepared according to Example 1 to 39mL of dimethyl sulfoxide, heat to 100°C and stir to dissolve, slowly add distilled water in batches until it just appears turbid, a total of about 10.5mL, slowly lower to room temperature, Cool and crystallize for 8 hours, filter, wash the filter cake with ethanol, spin dry to obtain 10.3 g of light yellow powder, yield 79%, HPLC purity: 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com