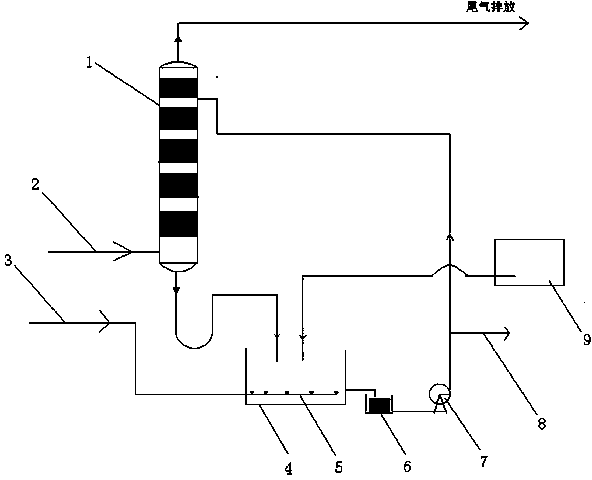
Method for preparing magnesium nitrate solution by using nitric acid tail gas and apparatus thereof
A nitric acid tail gas and magnesium nitrate technology, applied in the direction of magnesium nitrate, separation methods, chemical instruments and methods, etc., can solve the problems of reduced nitric acid production and increased ammonia consumption, and achieve high NO utilization, complete reaction, energy-saving and environmentally friendly production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A kind of method utilizing nitric acid tail gas to prepare magnesium nitrate solution, comprises the steps:
[0039] (1) After the nitric acid tail gas is washed by the washing tower, it is sprayed with water into the surface of magnesium oxide to form a solution; the pressure inside the washing tower is 0.03MPa, the temperature at the bottom is 30°C, and the temperature at the top is 20°C; the nitrogen in the tail gas discharged from the washing tower is The oxide content is less than 200PPm.
[0040] (2) Pass compressed air evenly into the solution at a temperature of 20°C in (1) for stirring to form a magnesium nitrate solution, and adjust its pH to be neutral;
[0041] (3) After the magnesium nitrate solution is filtered, it enters the washing tower and repeats the steps (1) and (2) to increase the concentration of the magnesium nitrate solution until the reaction is completed;
[0042] (4) Precipitate for 8 hours and filter to obtain the finished magnesium nitrate...
Embodiment 2
[0045] A kind of method utilizing nitric acid tail gas to prepare magnesium nitrate solution, comprises the steps:
[0046] (1) After the nitric acid tail gas is washed by the washing tower, it is sprayed with water into the surface of magnesium oxide to form a solution; the pressure inside the washing tower is 0.05MPa, the temperature at the bottom is 40°C, and the temperature at the top is 20°C; the nitrogen in the exhaust tail gas of the washing tower is The oxide content is less than 200PPm.
[0047] (2) Pass compressed air evenly into the solution at a temperature of 40°C in (1) for stirring to form a magnesium nitrate solution, and adjust its pH to be neutral;
[0048] (3) After the magnesium nitrate solution is filtered, it enters the washing tower and repeats the steps (1) and (2) to increase the concentration of the magnesium nitrate solution until the reaction is completed;
[0049] (4) Filter after 9 hours of precipitation to obtain the finished magnesium nitrate s...
Embodiment 3
[0052] A kind of method utilizing nitric acid tail gas to prepare magnesium nitrate solution, comprises the steps:
[0053] (1) After the nitric acid tail gas is washed by the washing tower, it is sprayed with water into the surface of magnesium oxide to form a solution; the pressure inside the washing tower is 0.08MPa, the temperature at the bottom is 60°C, and the temperature at the top is 50°C; the nitrogen in the tail gas discharged from the washing tower is The oxide content is less than 200PPm.
[0054] (2) Pass compressed air evenly into the solution at a temperature of 60°C in (1) for stirring to form a magnesium nitrate solution, and adjust its pH to be neutral;
[0055] (3) After the magnesium nitrate solution is filtered, it enters the washing tower and repeats the steps (1) and (2) to increase the concentration of the magnesium nitrate solution until the reaction is completed;
[0056] (4) Filter after 10 hours of precipitation to obtain the finished magnesium nit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com